AI Detects More Breast Cancers with Fewer False Positives
Released: June 04, 2024
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Imani Harris
1-630-481-1009
iharris@rsna.org
OAK BROOK, Ill. — Using artificial intelligence (AI), breast radiologists in Denmark have improved breast cancer screening performance and reduced the rate of false-positive findings. Results of the study were published today in Radiology, a journal of the Radiological Society of North America (RSNA).
Mammography successfully reduces breast cancer mortality, but also carries the risk of false-positive findings. In recent years, researchers have studied the use of AI systems in screening.
"We believe AI has the potential to improve screening performance," said Andreas D. Lauritzen, Ph.D., a post-doctoral student at the University of Copenhagen and researcher at Gentofte Hospital in Denmark.
When used to triage likely normal screening results or assist with decision support, AI also can substantially reduce radiologist workload.
"Population-based screening with mammography reduces breast cancer mortality, but it places a substantial workload on radiologists who must read a large number of mammograms, the majority of which don't warrant a recall of the patient," Dr. Lauritzen said. "The reading workload is further compounded when screening programs employ double reading to improve cancer detection and decrease false-positive recalls."
Dr. Lauritzen and colleagues set out to compare workload and screening performance in two cohorts of women who underwent screening before and after AI implementation.
The retrospective study compared two groups of women between the ages of 50 and 69 who underwent biennial mammography screening in the Capital Region of Denmark.
In the first group, two radiologists read the mammograms of women screened between October 2020 and November 2021 before the implementation of AI. The screening mammograms of the second group of women performed between November 2021 and October 2022 were initially analyzed by AI. Mammograms deemed likely to be normal by AI were then read by one of 19 specialized full-time breast radiologists (called a single-read). The remaining mammograms were read by two radiologists (called a double-read) with AI-assisted decision support.
The commercially available AI system used for screening was trained by deep learning models to highlight and rate suspicious lesions and calcifications within mammograms. All women who underwent mammographic screening were followed for at least 180 days. Invasive cancers and ductal carcinoma in situ (DCIS) detected through screening were confirmed through needle biopsy or surgical specimens.
In total, 60,751 women were screened without AI, and 58,246 women were screened with the AI system. In the AI implementation group, 66.9% (38,977) of the screenings were single-read, and 33.1% (19,269) were double-read with AI assistance.
Compared to screening without AI, screening with the AI system detected significantly more breast cancers (0.82% versus 0.70%) and had a lower false-positive rate (1.63% versus 2.39%).
"In the AI-screened group, the recall rate decreased by 20.5 percent, and the radiologists' reading workload was lowered by 33.4 percent," Dr. Lauritzen said.
The positive predictive value of AI screening was also greater than that of screening without AI (33.5% versus 22.5%). In the AI group, a higher proportion of invasive cancers detected were 1 centimeter or less in size (44.93% vs. 36.60%).
"All screening performance indicators improved except for the node-negative rate which showed no evidence of change," Dr. Lauritzen said.
Dr. Lauritzen said more research is needed to evaluate long-term outcomes and ensure overdiagnosis does not increase.
"Radiologists typically have access to the women's previous screening mammograms, but the AI system does not," he said. "That's something we'd like to work on in the future."
It is also important to note that not all countries follow the same breast cancer screening protocols and intervals. U.S. breast cancer screening protocols differ from protocols used in Denmark.
"Early Indicators of the Impact of Using AI in Mammography Screening for Breast Cancer." Collaborating with Dr. Lauritzen were Martin Lillholm, Ph.D., Elsebeth Lynge, Ph.D., Mads Nielsen, Ph.D., Nico Karssemeijer, Ph.D., and Ilse Vejborg, M.D.
Radiology is edited by Linda Moy, M.D., New York University, New York, N.Y., and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology)
RSNA is an association of radiologists, radiation oncologists, medical physicists and related scientists promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Illinois. (RSNA.org)
For patient-friendly information on breast cancer screening, visit RadiologyInfo.org.
Video (MP4):

Video 1. Andreas D. Lauritzen, Ph.D., discusses his research on using AI to improve breast cancer screening performance and reducing the rate of false-positive findings.
Download MP4
(Right-click and Save As)
Images (JPG, TIF):
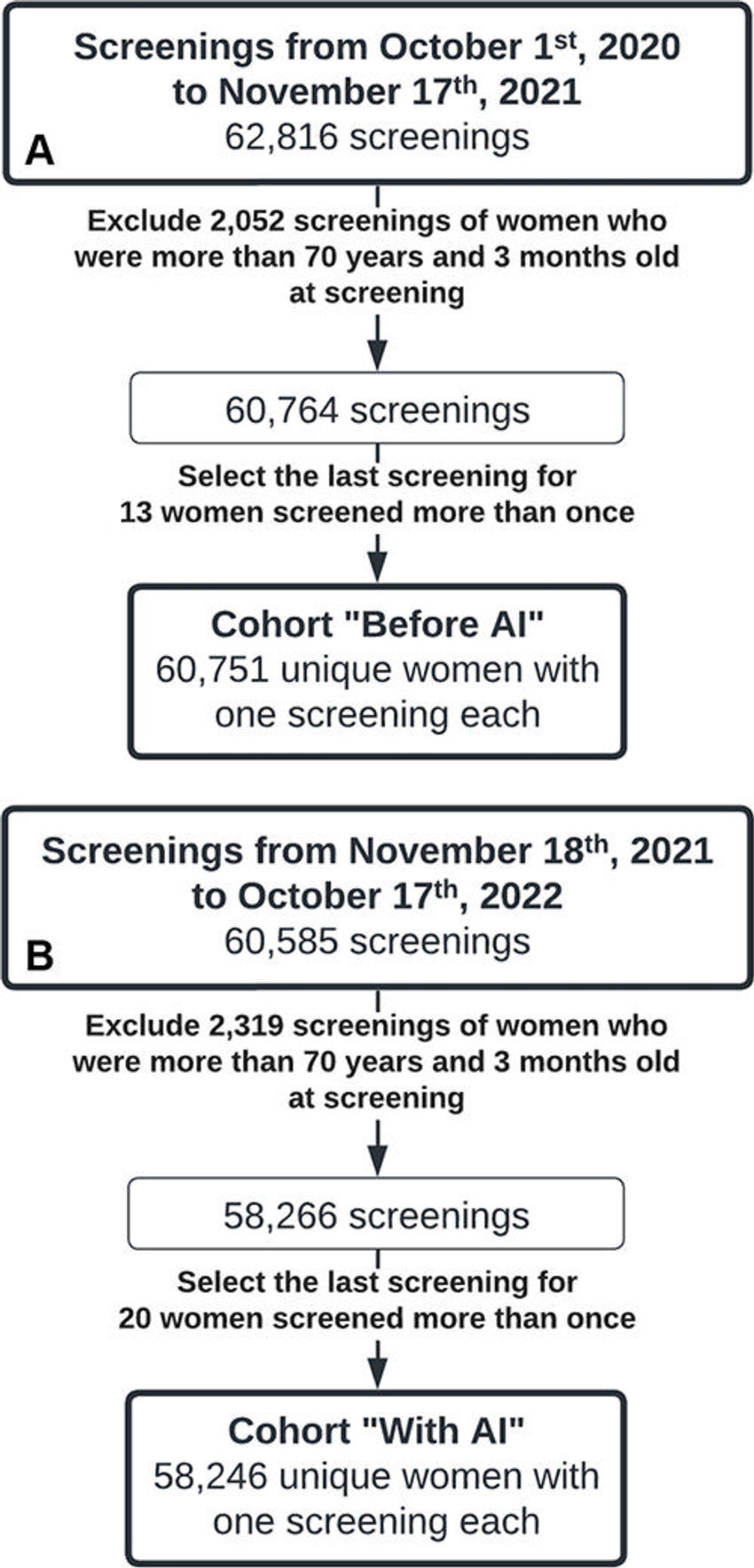
Figure 1. Flow diagram of the inclusion and exclusion process for the cohort of women screened (A) before the implementation of an artificial intelligence (AI) system and (B) after the AI system was implemented for mammography screening.
High-res (TIF) version
(Right-click and Save As)

Figure 2. Flow diagram depicts mammography reading protocols (A) before an artificial intelligence (AI) system was implemented in screening and (B, C) after the AI system was implemented with (B) the original (before May 3, 2022) or (C) a higher (on or after May 3, 2022) AI examination score threshold for selecting screenings for single reading. Green boxes indicate likely normal screenings selected for single reading; yellow boxes indicate screenings selected for AI-assisted double reading, in which case radiologists (*) had access to decision support in the form of highlighted lesions provided by the AI system. neg. = negative, pos. = positive.
High-res (TIF) version
(Right-click and Save As)
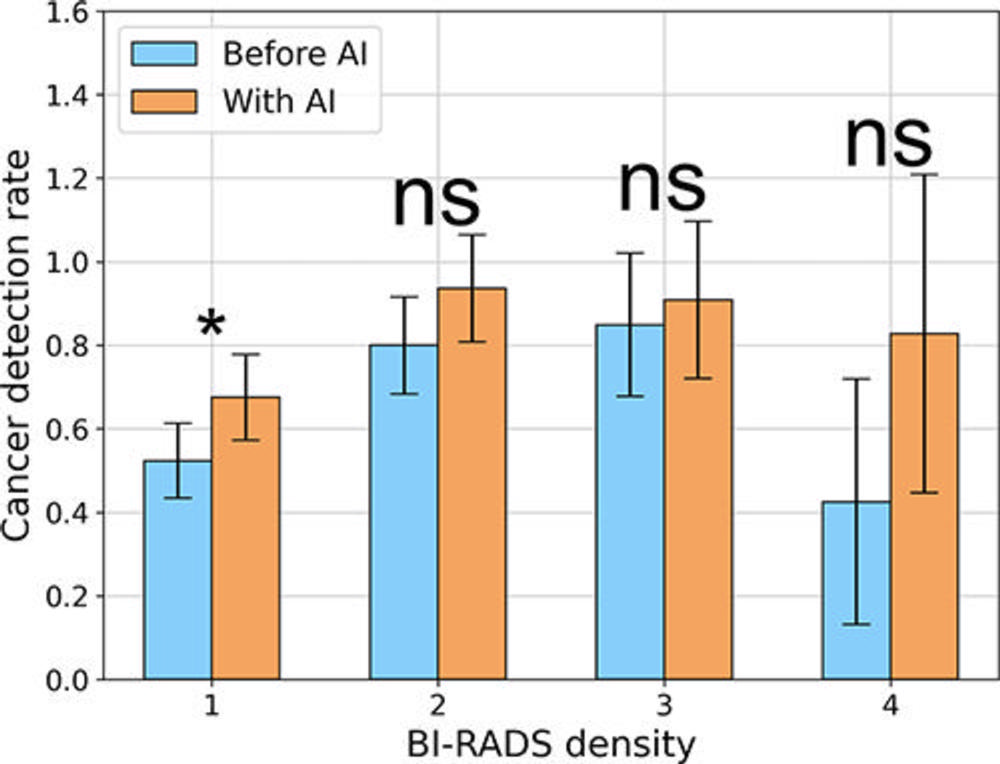
Figure 3. Cancer detection rates across Breast Imaging Reporting and Data System (BI-RADS) density categories, as assigned by the second senior radiologist. Error bars indicate 95% CIs. * = P < .05. AI = artificial intelligence, ns = not significant.
High-res (TIF) version
(Right-click and Save As)
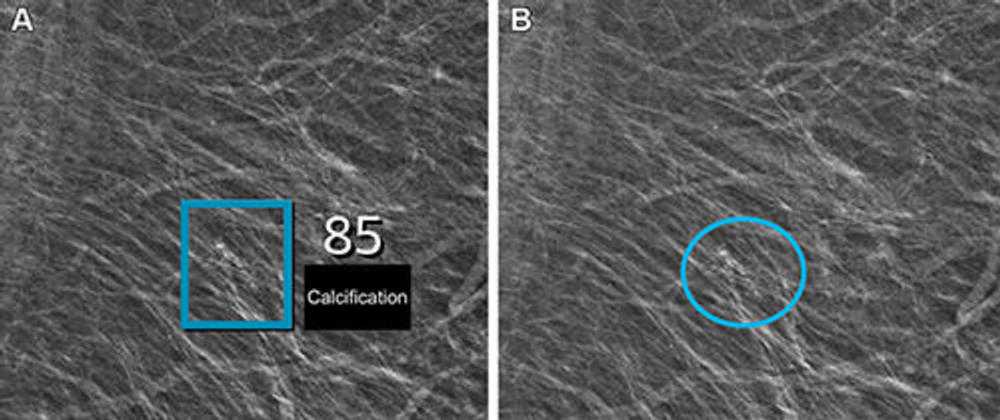
Figure 4. Left mediolateral oblique full-field digital mammographic view in a 67-year-old woman with a Breast Imaging Reporting and Data System density of 1 who underwent screening with the artificial intelligence (AI) system. (A) Image shows AI-provided marking (square). The screening received a high AI examination score of 10, based on this area with arterial calcifications being given a score of 85 out of 100 by the AI system. (B) Same image as in A, but with findings by the radiologists. Because of the high AI examination score, the screening was double read by two radiologists, who determined that the arterial calcifications (circle) did not yield suspicions for breast cancer. The woman was not recalled for diagnostic assessment.
High-res (TIF) version
(Right-click and Save As)
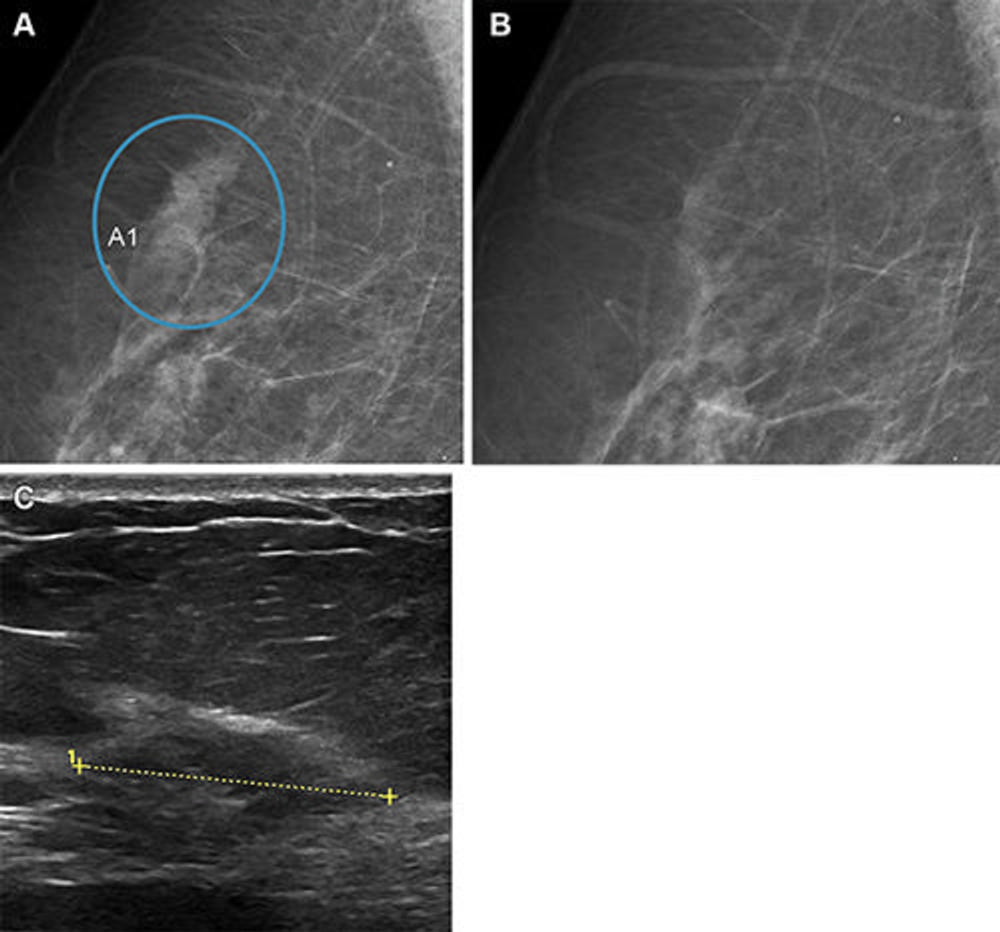
Figure 5. Images in a woman who had a Breast Imaging Reporting and Data System density of 1 and was 62 years old when she underwent screening with the artificial intelligence (AI) system. The screening received a low AI examination score of 2; therefore, a single read was conducted by a senior radiologist. The AI system did not detect any suspicious lesions. (A) Right mediolateral oblique full-field digital mammographic view shows suspicious lesion (circle, A1) identified by the senior radiologist. (B) Matching view from the prior screening round shows that the lesion was not visible at that time. The tissue was considerably changed in appearance at the time of the study screening, which led to recall. (C) Cropped US image shows a 25-mm invasive ductal carcinoma (line) that was observed during the diagnostic workup.
High-res (TIF) version
(Right-click and Save As)
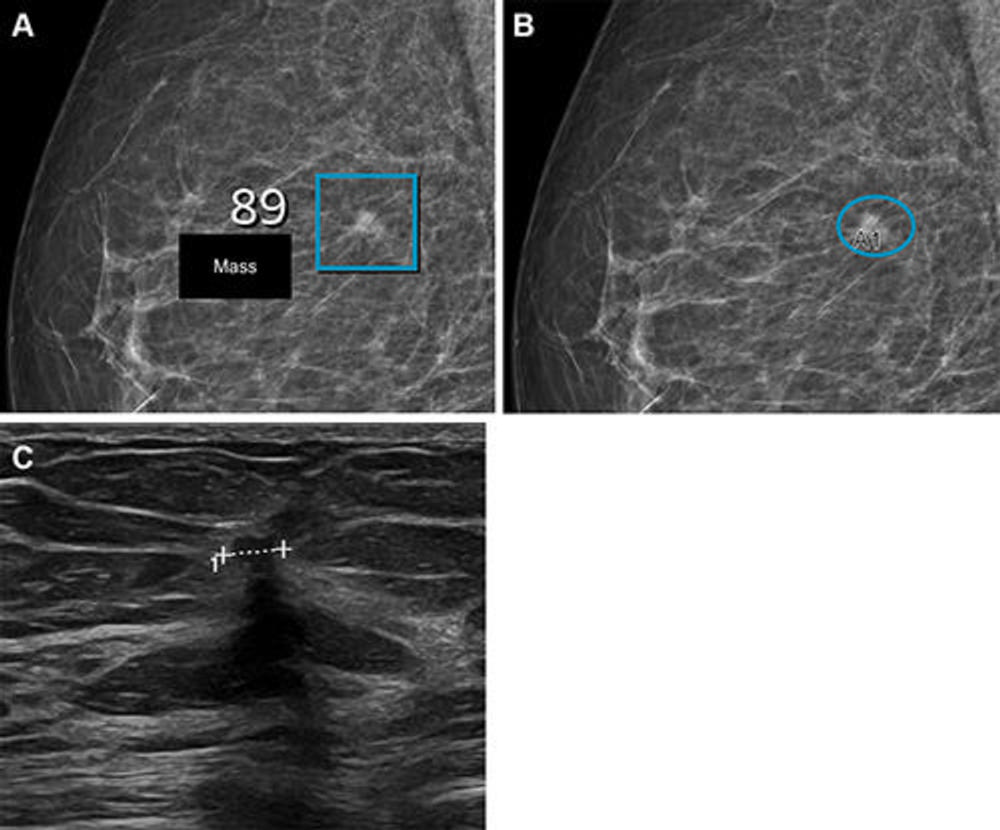
Figure 6. Images in a woman who had a Breast Imaging Reporting and Data System density of 2 and was 57 years old when she underwent screening with the artificial intelligence (AI) system. (A) Right mediolateral oblique full-field digital mammographic view shows AI-provided marking (square). The screening received a high AI examination score of 10 based on this lesion with a score of 89 out of 100. (B) Same image as in A, but with findings by the radiologists. Because of the high AI examination score, the screening was double read by two radiologists, who marked the same lesion (oval, A1) as the AI system, which led to recall. (C) Cropped US image shows a small (4 × 7 mm) invasive carcinoma (line) that was observed during the diagnostic workup.
High-res (TIF) version
(Right-click and Save As)
