RSNA Publishes New QIBA Profile for Knee Cartilage MRI
Released: September 07, 2021
At A Glance
- An RSNA committee has developed a new QIBA profile to provide more reliable, reproducible results for MRI-based measurements of knee cartilage degeneration.
- The new profile is an important step in developing early detection of cartilage abnormalities when treatment is effective.
- The committee developed the profile to help slow down disease and prevent progression to irreversible osteoarthritis.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Katherine Anderson
1-630-491-1009
kanderson@rsna.org
OAK BROOK, Ill. — New recommendations will help provide more reliable, reproducible results for MRI-based measurements of cartilage degeneration in the knee, helping to slow down disease and prevent progression to irreversible osteoarthritis, according to a special report published in the journal Radiology.
Osteoarthritis is the most common type of arthritis, with a prevalence of more than 33% in adults older than 65 years. It exacts a major toll on society in the form of costs related to pain, disability and reduced quality of life. There is currently no way to cure or reverse it.
"By the time there is structural damage to the cartilage, treatment choices are very limited," said paper coauthor Majid Chalian, M.D., assistant professor of radiology and section head of musculoskeletal imaging and intervention in the Department of Radiology at the University of Washington in Seattle. "We cannot treat the damaged cartilage, and we cannot prevent osteoarthritis because the cartilage is not going to regrow."
MRI-based cartilage compositional analysis is a promising tool for revealing biochemical and microstructural changes in the early stages of osteoarthritis. Two advanced MRI techniques, T1rho and T2 mapping, have been established for assessing cartilage composition. T2 mapping is the only one currently available commercially.
While the techniques are promising, clinical applications have been limited.
"The issue with these compositional cartilage imaging measurements is that the reliability and reproducibility of the numbers are not great," Dr. Chalian said. "They can be different from one scanner to another or when you use the same scanner at different times."
To address these shortcomings, Dr. Chalian and his colleagues from the Radiological Society of North America's (RSNA) Musculoskeletal Biomarkers Committee of the Quantitative Imaging Biomarkers Alliance (QIBA) collaborated to create a QIBA profile for cartilage compositional imaging. They analyzed major publications in the field and made several important determinations.
First, they found that cartilage T1rho and T2 values are measurable with 3T MRI with a variation of 4% to 5%.
"This is very important because it could expedite clinical trials, allowing them to be based on a smaller sample size," Dr. Chalian said.
The committee also determined that a measured increase/decrease in T1rho and T2 value of 14% or more indicates a minimum detectable change, which can be used for defining response/progression criteria for quantitative cartilage imaging. If only an increase in T1rho and T2 is expected, as is the case with progressive cartilage degeneration, then an increase of 12% represents a minimum detectable change.
"There was no generally acceptable cut-off for T1rho and T2 values for research and clinical use," Dr. Chalian said. "Now we can say that if you image one knee and then do follow-up imaging in two years using the same technique, and you have a 14% change in T1rho and T2 value of the cartilage, that's actually a meaningful change."
The new profile is an important step toward early detection of cartilage abnormalities when treatment is effective. Through lifestyle modification and therapeutic drugs, patients with knee pain and athletes recovering from injury could avoid structural damage and osteoarthritis.
"Based on these numbers, we can say whether the cartilage is abnormal and prone to degeneration," Dr. Chalian said.
Now that the profile has been generated, the researchers hope to facilitate clinical applications by promoting a move from manual segmentation of the MRI sequences to a much faster and more efficient automated approach. There are several machine learning applications for automatic cartilage segmentation that are promising, according to Dr. Chalian.
"We are hoping that a machine learning approach can segment out the cartilage, and then we can apply this profile on the segmented cartilage so that we can make things go fast in busy clinical settings," he said.
Launched by RSNA in 2007, QIBA aims to improve the value and practicality of quantitative imaging biomarkers by reducing variability across devices, sites, patients, and time and to unite researchers, health care professionals and industry to advance quantitative imaging and the use of imaging biomarkers in clinical trials and clinical practice.
"The QIBA Profile for MRI-based Compositional Imaging of Knee Cartilage." Collaborating with Dr. Chalian were Xiaojuan Li, Ph.D., Ali Guermazi, M.D., Ph.D., Nancy A. Obuchowski, Ph.D., John A. Carrino, M.D., M.P.H., Edwin H. Oei, M.D., and Thomas M. Link, M.D., Ph.D., for the RSNA QIBA MSK Biomarker Committee.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology)
RSNA is an association of radiologists, radiation oncologists, medical physicists and related scientists promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Illinois. (RSNA.org)
For patient-friendly information on musculoskeletal imaging, visit RadiologyInfo.org.
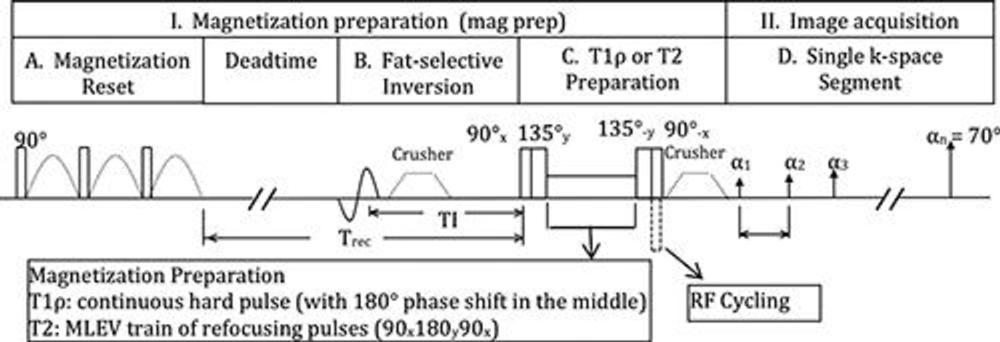
Figure 1. The magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots–based spin-lattice relaxation time constant in rotating frame (T1r) and T2 imaging sequence is available as a research prototype by the three major MRI vendors including GE Healthcare, Siemens Healthineers, and Philips Healthcare Solutions. MLEV = Malcolm Levitt composite-pulse decoupling sequence, RF = radiofrequency.
High-res (TIF) version
(Right-click and Save As)
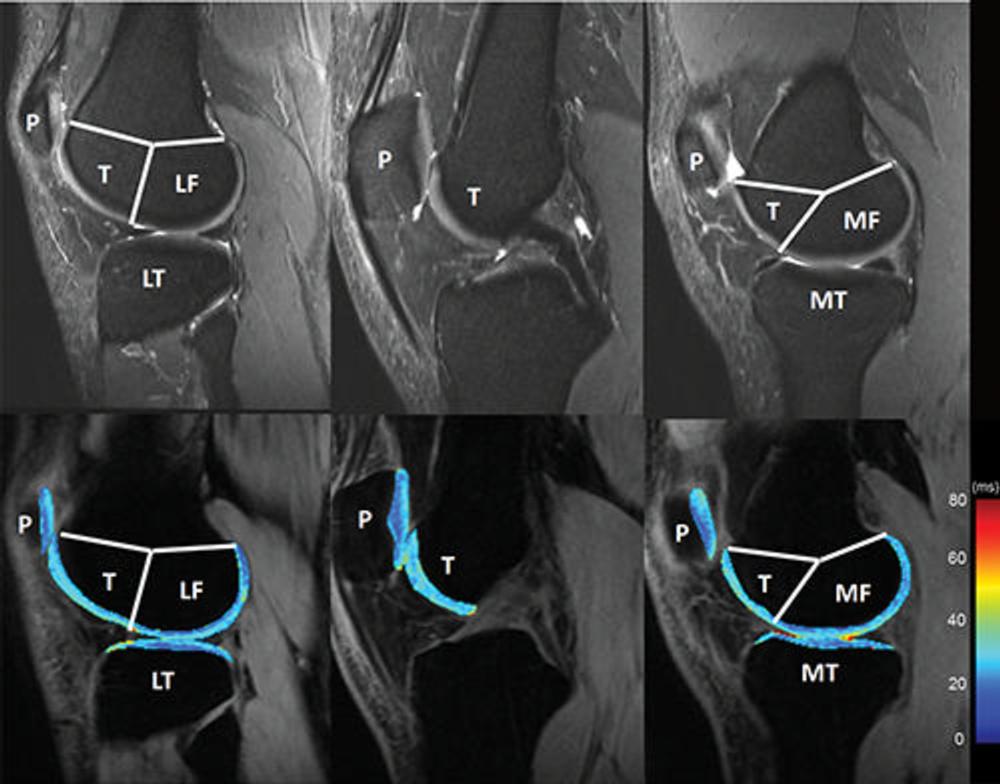
Figure 2. Knee cartilage compartments with anatomic labels implemented in lateral (left side), central (middle), and medial (right side) MRI obtained with an intermediate weighted fat-saturated fast-spin-echo sequence (top row) and a spin-lattice relaxation time constant in rotating frame (T1r) magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots sequence (bottom row, T1r maps). Study was performed without administration of intravenous gadolinium-based contrast material. The lateral femur (LF)/medial femur (MF) and lateral tibia (LT)/medial tibia (MT) can be further divided into subcompartments on the basis of meniscus anatomy according to Eckstein et al. P = patella, T = trochlea.
High-res (TIF) version
(Right-click and Save As)
