Ultrasound Technique Treats Prostate Cancer with Minimal Side Effects
Released: February 02, 2021
At A Glance
- MRI-guided high-intensity focused ultrasound effectively treats prostate cancer with minimal side effects.
- In focal therapy, an ultrasound transducer focuses sound waves to generate heat at a single point within the body and destroy the target tissue.
- Treatment was successfully completed with no major adverse events, with 41 of 44 participants disease-free at the treatment site five months later.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org
OAK BROOK, Ill. — A technique that delivers high-intensity focused ultrasound to targeted tissue under MRI guidance effectively treats intermediate-risk prostate cancer with minimal side effects, according to a study published in Radiology.
Prostate cancer is the most common cancer among men, aside from non-melanoma skin cancers. Common treatments to the entire gland, such as surgery and radiation therapy, are effective in eliminating the cancer, but they often leave patients with incontinence and sexual dysfunction.
A class of treatments called focal therapy offers an alternative for some men with intermediate-risk disease that is still confined to the prostate. In focal therapy, the cancer is ablated, or destroyed, by either heating or freezing the target tissue. Since the treatment is targeted to a small area within the prostate, side effects are generally less significant than those associated with surgery and radiation therapy.
High-intensity focused ultrasound is an example of focal therapy in which an ultrasound transducer focuses sound waves to generate heat at a single point within the body and destroy the target tissue. In the past, it has been performed under ultrasound guidance, but ultrasound does not visualize the site of cancer within the prostate gland well enough to allow for a targeted approach.
For the new study, researchers studied a device that delivers MRI-guided focused ultrasound (MRgFUS). While the patient is under general anesthesia, a probe is placed in the rectum that focuses high-frequency ultrasonic waves to the site of the cancer. The procedure takes approximately four hours to perform.
"By combining the high-intensity focused ultrasound device with MRI, we can target our treatment to the exact location, because we're able to pinpoint precisely where the tumor is," said the study's principal investigator and lead author Sangeet Ghai, M.D., at Toronto's Joint Department of Medical Imaging, part of the University Health Network (UHN) Sinai Health and Women's College Hospital.
Dr. Ghai and colleagues from UHN and Sinai Health performed MRgFUS on 44 men with prostate cancer and tracked their outcomes using MRI, biopsies and surveys of erectile and urinary function.
Treatment was successfully completed in all the men. There were no major treatment-related adverse events, and 41 of 44 participants, or 93%, were disease-free at the treatment site on five-month biopsy.
Scores for erectile function and prostate symptoms were similar at baseline and five months.
"The results so far have been very good," Dr. Ghai said. "We treated a smaller area using this device, yet still had very good results. At the same time the patients preserved their erectile and urinary function."
Although MRgFUS requires additional expertise, resources and cost, it has several key advantages over other approaches. Use of MRI allows for thermal feedback during treatment, an important consideration since killing the cancerous tissue requires a temperature of more than 60 degrees Celsius.
"MRI almost instantaneously gives feedback as to the temperature that we've been able to achieve at the site," Dr. Ghai said. "If the temperature was not what I wanted to get, I can reheat that area so that chances for successful treatment increase."
An additional benefit of MRI is that it can show if there is any remaining vascularity in the treatment area, a sign that not all the cancer has been eradicated.
Pending approval from the U.S Food and Drug Administration and Health Canada, MRgFUS has promise as an option for intermediate-risk patients who want their prostate cancer eradicated without diminishing their quality of life, a group that makes up a potentially significant number of patients. Dr. Ghai estimates that of all the prostate cancer patients who go on to get surgery or radiation therapy, approximately 20% to 30% would be eligible for some type of focal therapy like MRgFUS.
As part of the study, the researchers are collecting two-year follow-up data from all patients in the study group and plan to publish the results in another manuscript.
"MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial." Collaborating with Dr. Ghai were Antonio Finelli, M.D., FRCSC, Kateri Corr, B.Sc., Rosanna Chan, M.R.T.(MR)(N), Sarah Jokhu, H.B.Sc., C.C.R.P., Xuan Li, Ph.D., Stuart McCluskey, M.D., Ph.D., FRCPC, Anna Konukhova, Ph.D., Eugen Hlasny, M.R.T.(MR), Theodorus H. van der Kwast, M.D., Ph.D., FRCPC, Peter F. Incze, M.D., FRCSC, Alexandre R. Zlotta, M.D., Ph.D., FRCSC, Robert J. Hamilton, M.D., M.P.H., FRCSC, Masoom A. Haider, M.D., FRCPC, Walter Kucharczyk, M.D., FRCPC, and Nathan Perlis, M.D., M.Sc., FRCSC.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology)
RSNA is an association of radiologists, radiation oncologists, medical physicists and related scientists promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Illinois. (RSNA.org)
For patient-friendly information on prostate imaging, visit RadiologyInfo.org.
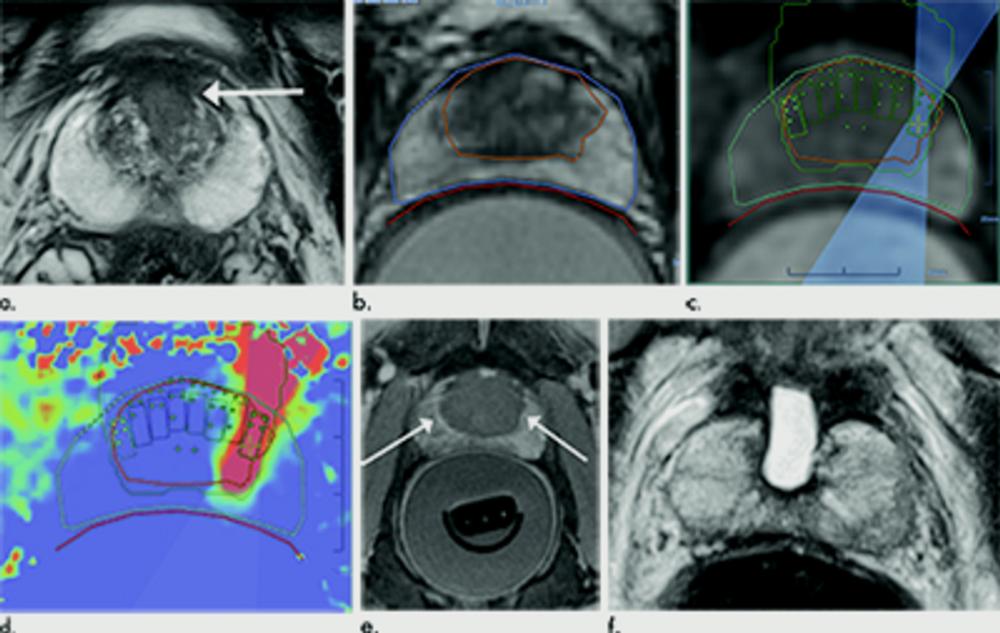
Figure 1. Images in 69-year-old man with biopsy-confirmed Gleason score 7 (3+4) prostate cancer. (a) Pretreatment axial T2-weighted fast spin-echo MRI scan (repetition time msec/echo time msec, 3820/97) shows tumor in midline anterior transition zone (arrow). (b) Intraoperative MRI scan shows contoured rectal wall (red line), prostate margin (blue outline), and region of interest (orange outline). Because the urethra was included in planned treatment volume, a suprapubic catheter was placed for continuous bladder drainage during treatment. (c) Intraoperative MRI scan shows focused ultrasound beam path (blue) overlaid on treatment plan. Rectangles illustrate each sonication spot. (d) Thermal map image obtained during treatment with heat deposition color coded in red overlaid on sonication spot. (e) Axial gadopentetate dimeglumine–enhanced MRI scan (230/2.97) obtained immediately after treatment shows devascularized ablated volume (arrows). (f) Corresponding T2-weighted fast spin-echo MRI scan (3820/97) at 5 months after ablation shows complete involution of transition zone. All seven cores from treatment area margins were negative for cancer at biopsy.
High-res (TIF) version
(Right-click and Save As)
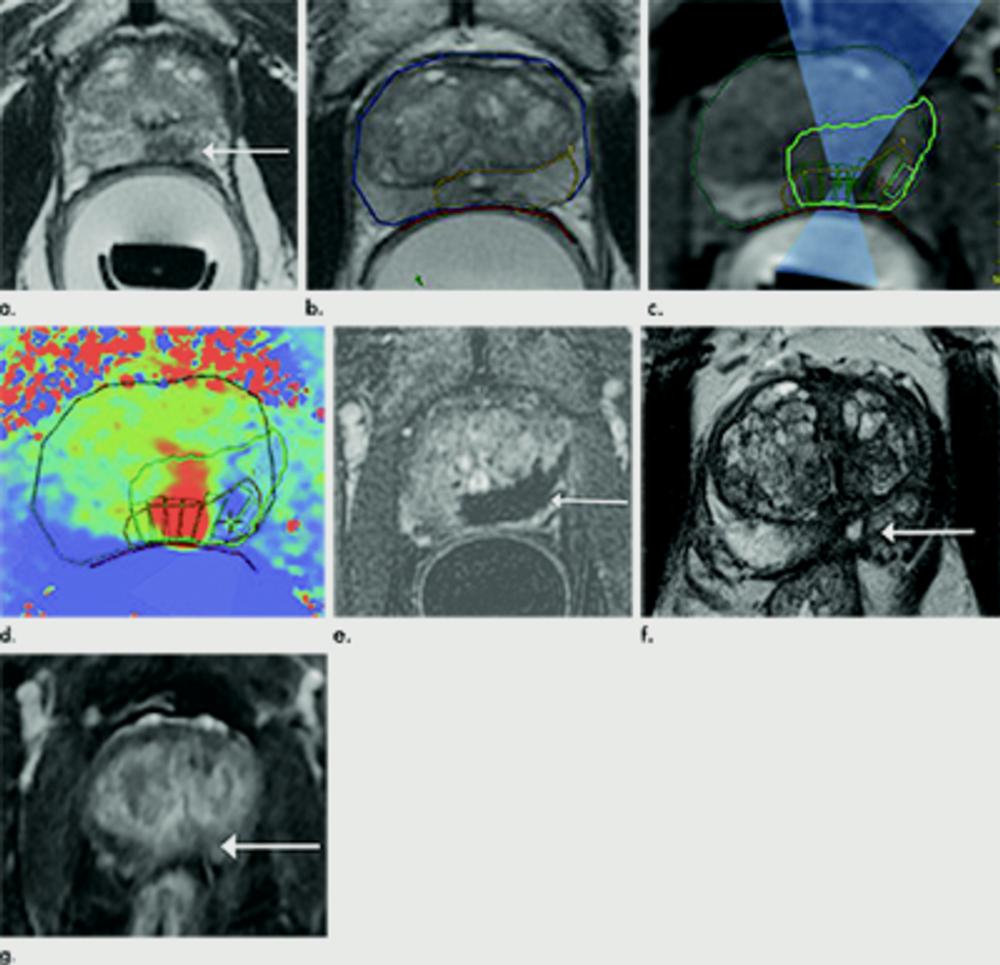
Figure 2. Images in 75-year-old man with biopsy-confirmed Gleason score 7 (4+3) prostate cancer. (a) Pretreatment axial T2-weighted fast spin-echo MRI scan on day of treatment (repetition time msec/echo time msec, 4416/81) shows tumor in left posteromedial peripheral zone at midgland (arrow). Endorectal treatment probe with degassed water is seen in rectum. (b) Intraoperative MRI scan shows contoured rectal wall (red line), prostate margin (blue outline), and region of interest (yellow outline). Because urethra was included in planned treatment volume, a suprapubic catheter was placed for continuous bladder drainage during treatment. (c) Intraoperative MRI scan shows focused ultrasound beam path (blue) overlaid on treatment plan. Rectangles illustrate each sonication spot. (d) Thermal map image obtained during treatment with heat deposition color coded in red overlaid on sonication spot. (e) Axial gadopentetate dimeglumine–enhanced subtraction MRI scan (200/5.4) obtained immediately after treatment shows devascularized ablated volume (arrow). Note that devascularized area does not extend into transition zone anteriorly. (f) Corresponding axial T2-weighted fast spin-echo MRI scan (4140/97) at 5 months after ablation shows scarring at ablation site (arrow). (g) Axial gadopentetate dimeglumine–enhanced subtraction MRI scan (5.39/1.88) does not show any early enhancement on dynamic contrast-enhanced images at treatment area (arrow). All six cores from treatment area, including margins, were negative for cancer at biopsy.
High-res (TIF) version
(Right-click and Save As)

Figure 3. Flowchart of participant enrollment. csPCa = clinically significant prostate cancer, GG = grade group, IQR = interquartile range, PCa = prostate cancer.
High-res (TIF) version
(Right-click and Save As)

Figure 4. Sexual function results after MRI-guided focused ultrasound for unifocal localized intermediate-risk prostate cancer, measured with International Index of Erectile Function-15 (IIEF-15) questionnaire. Graph obtained with generalized estimating equation statistical model shows mean of IIEF-15 scores across time between groups of treatments with nonperfused volume (NPV) of less than 15 cm3 (n = 23) and 15 cm3 or greater (n = 4) among participants who were sexually active at baseline (adjusted P = .30). Mean ± standard deviation of NPV less than 15 cm3 was 63.9 ± 8.3 at baseline, 50.7 ± 17.7 at 6 weeks, and 57.6 ± 15.2 at 5 months. Mean ± standard deviation of NPV greater than 15 cm3 was 63.2 ± 6.7 at baseline, 12.8 ± 5.6 at 6 weeks, and 30.8 ± 20.1 at 5 months.
High-res (TIF) version
(Right-click and Save As)
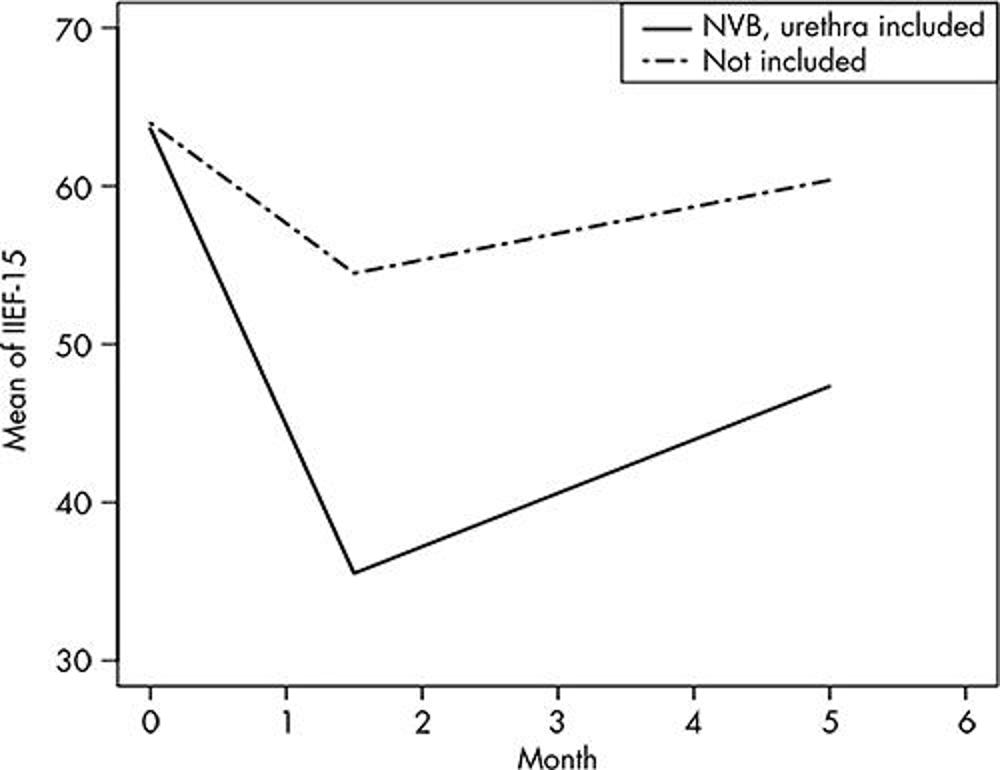
Figure 5. Sexual function results after MRI-guided focused ultrasound for unifocal localized intermediate-risk prostate cancer, measured with International Index of Erectile Function-15 (IIEF-15) questionnaire. Graph obtained with generalized estimating equation statistical model shows mean of IIEF-15 scores across time between groups of participants undergoing treatments where unilateral neurovascular bundle (NVB), urethra, or both were included in the treatment volume (n = 14) versus those participants in whom these structures were spared (n = 13), among men who were sexually active at baseline (adjusted P > .99). Mean ± standard deviation of NVB, urethra included, was 63.6 ± 6.9 at baseline, 35.5 ± 22.8 at 6 weeks, and 47.4 ± 22.6 at 5 months. Mean ± standard deviation of NVB, urethra not included, was 64 ± 9.3 at baseline, 54.4 ± 15.8 at 6 weeks, and 60.4 ± 8.8 at 5 months.
High-res (TIF) version
(Right-click and Save As)
