New Research Highlights Blood Clot Dangers of COVID-19
Released: April 23, 2020
At A Glance
- New research highlights the risk of COVID-19 blood clot complications and offers guidance for prevention, diagnosis and treatment.
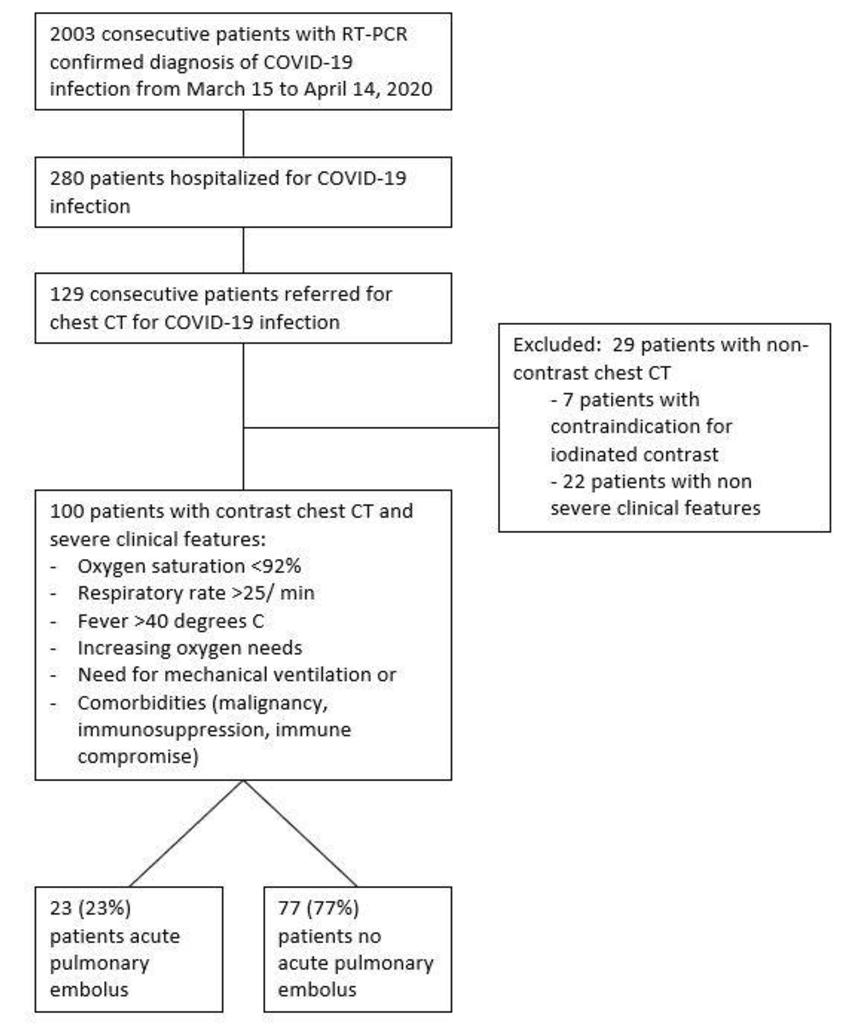
- Two studies from France found pulmonary emboli in 23-30% of COVID-19 patients.
- Findings suggest that that microvascular thrombotic processes may play a role in respiratory failure in patients with COVID-19.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Dionna Arnold
1-630-590-7791
darnold@rsna.org
OAK BROOK, Ill. (April 23, 2020) — A special report published today in the journal Radiology outlines prevention, diagnosis and treatment of complications stemming from blood clots in patients with COVID-19. The journal also published two research letters and a case study on this topic.
Clinicians worldwide face this new severe infectious lung disease with no proven therapies. Based on recent reports that demonstrated a strong association between elevated D-dimer levels and poor prognosis, concerns have risen about thrombotic complications in patients with COVID-19.
The National Institute for Public Health of the Netherlands asked a group of radiology and vascular medicine experts to provide guidance for the imaging workup and treatment of these important complications. Their report summarizes evidence for thromboembolic disease and potential diagnostic and preventive actions that can be taken.
“Worldwide, COVID-19 is being treated as a primary pulmonary disease,” said Edwin J.R. van Beek, M.D., Ph.D., director at Edinburgh Imaging, Queens Medical Research Institute, at the University of Edinburgh, U.K. “From the analysis of all available current medical, laboratory and imaging data on COVID-19, it became clear that symptoms and diagnostic tests could not be explained by impaired pulmonary ventilation alone.”
Recent observations suggest that respiratory failure in COVID-19 is not driven by the development of the acute respiratory distress syndrome alone, but that microvascular thrombotic processes may play a role. This may have important consequences for the diagnostic and therapeutic management of these patients. There is a strong association between D-dimer levels, disease progression and chest CT features suggesting venous thrombosis. In addition, various studies in patients with COVID-19 have shown a very strong association between increased D-dimer levels and severe disease/poor prognosis.
The report authors stress that careful attention needs to be paid to the initial diagnosis and treatment of the prothrombotic and thrombotic state that can occur in a substantial percentage of COVID-19 patients.
“Imaging and pathological investigations confirmed the COVID-19 syndrome is a thrombo-inflammatory process that initially affects lung perfusion, but consecutively affects all organs of the body,” Professor van Beek said. “This highly thrombotic syndrome leads to macro-thrombosis and embolism. Therefore, strict thrombosis prophylaxis, close laboratory and appropriate imaging monitoring with early anti-coagulant therapy in case of suspected venous thromboembolism are indicated.”
Recommendations for diagnostic and therapeutic management, which vary based on patient symptoms and risk profiles, include prophylactic-dose heparin, chest CT, CT pulmonary angiography and routine D-dimer testing.
Findings have also emerged linking COVID-19 more specifically with pulmonary embolism. A research letter from Hôpitaux Universitaires de Strasbourg published today in Radiology reported that of 106 pulmonary CT angiograms performed for COVID-19 patients over a one-month period in a tertiary care center in France, 32 patients (30%) had acute pulmonary embolus (PE). This rate of PE is much higher than usually encountered in critically ill patients without COVID-19 infection (1.3%,) or in emergency department patients (3 to 10%). In the study, a D-dimer threshold of 2660 μg/L detected all patients with PE on chest CT.
A second research letter published today described a study from Centre Hospitalier Universitaire de Besancon in France pointed to high proportion (23%) of COVID-19 patients with contrast CT had PE. PE was diagnosed at mean of 12 days from symptom onset. Patients with PE were more likely require care in the critical care unit and to require mechanical ventilation.
Lastly, a case report from Cooper University Hospital in Camden, New Jersey, describes multiple areas of pulmonary and arterial thrombosis in an 84-year-old man with COVID-19.
“COVID-19 is more than a lung infection,” Professor van Beek said. “It affects the vasculature of the lungs and other organs and has a high thrombosis risk with acute life-threatening events that require adequate treatment with anticoagulants based on laboratory monitoring with appropriate imaging tests as required.”
RSNA is committed to connecting radiologists and the radiology community to the most timely and useful COVID-19 information and resources. RSNA’s COVID-19 Resources page houses the latest guidance, original research, image collection and more. The page will be updated on an ongoing basis.
“Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands.” Matthijs Oudkerk, Harry R. Büller, Dirkjan Kuijpers, Nick van Es, Sitse F. Oudkerk, Theresa C. McLoud, M.D., Diederik Gommers, Jaap van Dissel, Hugo ten Cate, Edwin J. van Beek, M.D., Ph.D.
“Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to D-Dimer Levels.” Ian Leonard-Lorant, M.D., Xavier Delabranche, M.D., Ph.D., François Severac, M.D., Juliec Helms, M.D., Ph.D., Coralie Pauzet, M.D., Olivier Collange, M.D., Ph.D., Françis Schneider, M.D., Ph.D., Aissam Labani, M.D., Pascal Bilbault, M.D., Ph.D., Sébastien Moliere, M.D., Pierre Leyendecker, M.D., Catherine Roy, M.D., and Mickaël Ohana, M.D., Ph.D.
“Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography.” Franck Grillet, M.D., Julien Behr, M.D., Paul Calame, M.D., Sébastien Aubry, M.D., Ph.D., Eric Delabrousse, M.D., Ph.D.
“Pulmonary, Cerebral, and Renal Thromboembolic Disease Associated with COVID-19 Infection.” Nadia Lushina, M.D., John S. Kuo, M.D., Hamza A. Shaikh, M.D.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology)
RSNA is an association of radiologists, radiation oncologists, medical physicists and related scientists promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Ill. (RSNA.org)
For patient-friendly information on blood clots, visit RadiologyInfo.org.
Images (JPG, TIF):

Figure 1. Axial contrast-enhanced CT of the chest. A, B, Diffuse bilateral ground-glass opacities and small bibasilar consolidations compatible with COVID-19 pneumonia. C, D, Filling defects consistent with pulmonary emboli within the right upper lobe, right middle lobe, right lower lobe, and left lower lobe pulmonary arteries (arrows). (Lushina, et al.)
High-res (TIF) version
(Right-click and Save As)

Figure 2. A, Axial contrast-enhanced CT of the chest demonstrates a filling defect in the aortic arch (arrow) consistent with thrombus. B, Coronal contrast-enhanced CT of the abdomen and pelvis demonstrates a wedge-shaped low-attenuation region in the superior pole of the left kidney (arrow) consistent with renal infarct. (Lushina, et al.)
High-res (TIF) version
(Right-click and Save As)
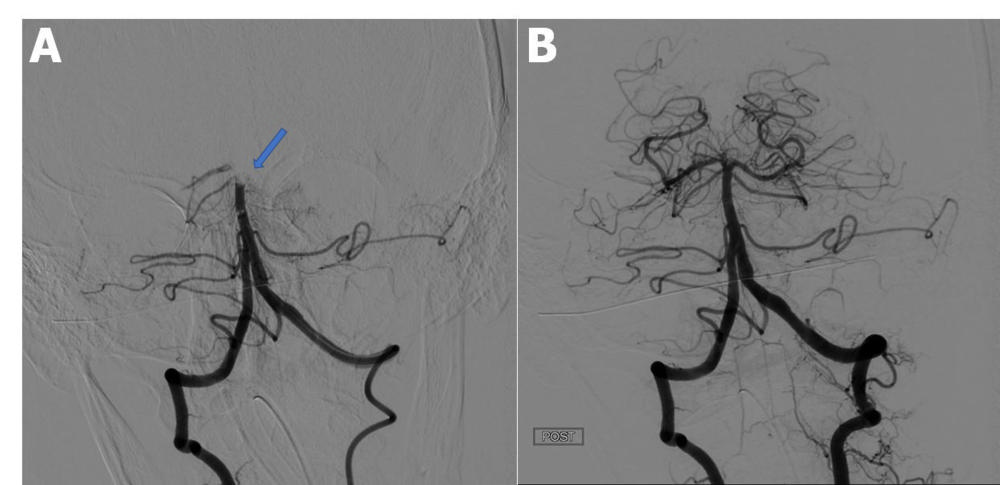
Figure 3. Catheter directed cerebral angiography. A, Pre-thrombectomy angiogram demonstrates an occluded distal basilar artery (arrow). B, Post-thrombectomy angiogram demonstrates successful restoration of the posterior circulation. (Lushina, et al.)
High-res (TIF) version
(Right-click and Save As)

Figure 5. Pulmonary CT angiography of a 68-year-old male. The CT scan was obtained 10 days after the onset of COVID-19 symptoms and on the day the patient was transferred to the intensive care unit. Axial CT images (lung windows) (a, b) show peripheral ground-glass opacities (arrow) associated with areas of consolidation in dependent portions of the lung (arrowheads). Interlobular reticulations, bronchiectasis (black arrow) and lung architectural distortion are present. Involvement of the lung volume was estimated to be between 25% and 50%. Coronal CT reformations (mediastinum windows) (c, d) show bilateral lobar and segmental pulmonary embolism (black arrows). (Grillet, et al.)
High-res (TIF) version
(Right-click and Save As)
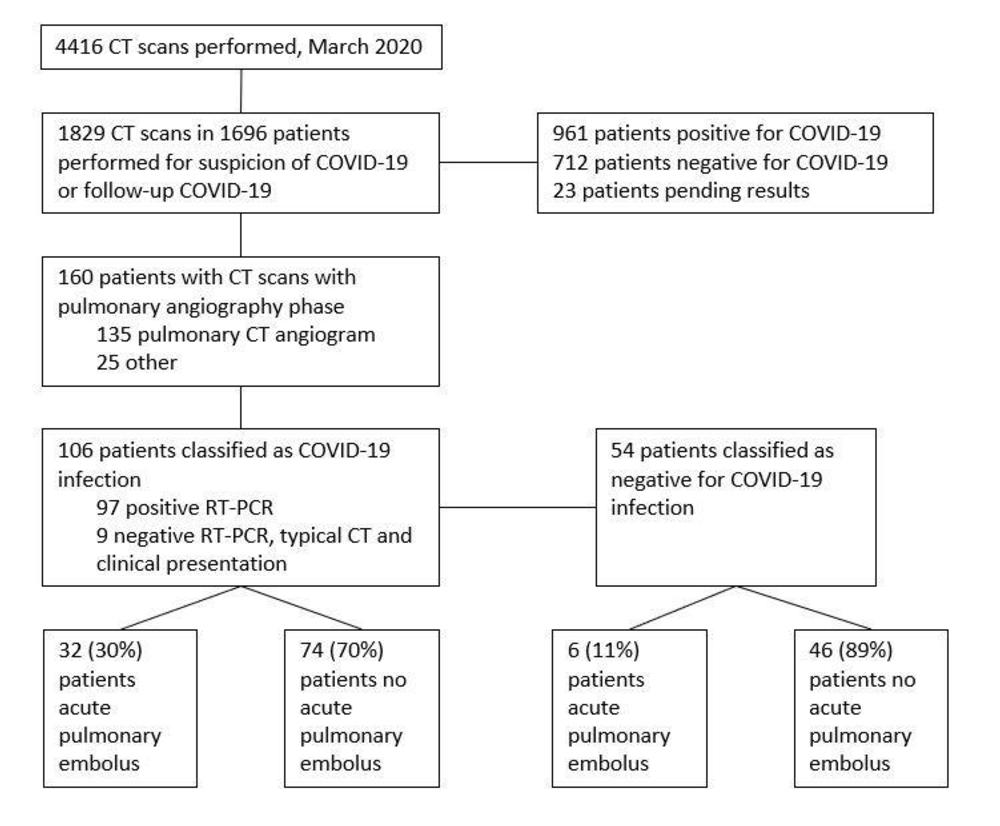
Figure 6. Flowchart of the study. (Leonard-Lorant, et al.)
High-res (TIF) version
(Right-click and Save As)
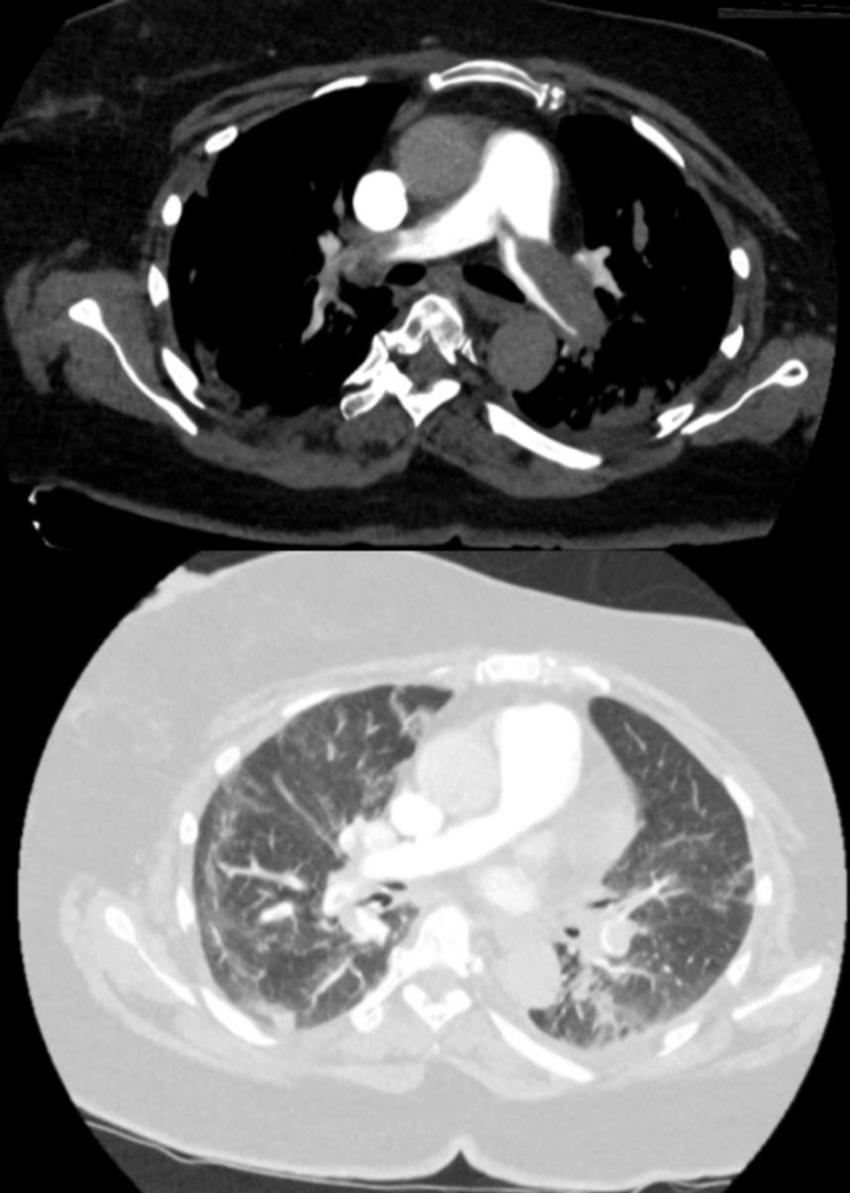
Figure 7. 71-year-old woman at day 3 of ICU stay for ARDS secondary to COVID-19. Pulmonary CT angiography was performed to investigate elevated of D-dimer value above 20 000 µg/L. The CT angiogram demonstrates bilateral filling defects in the main pulmonary arteries. Bilateral peripheral ground glass opacities and small areas of consolidation are present. (Leonard-Lorant, et al.)
High-res (TIF) version
(Right-click and Save As)
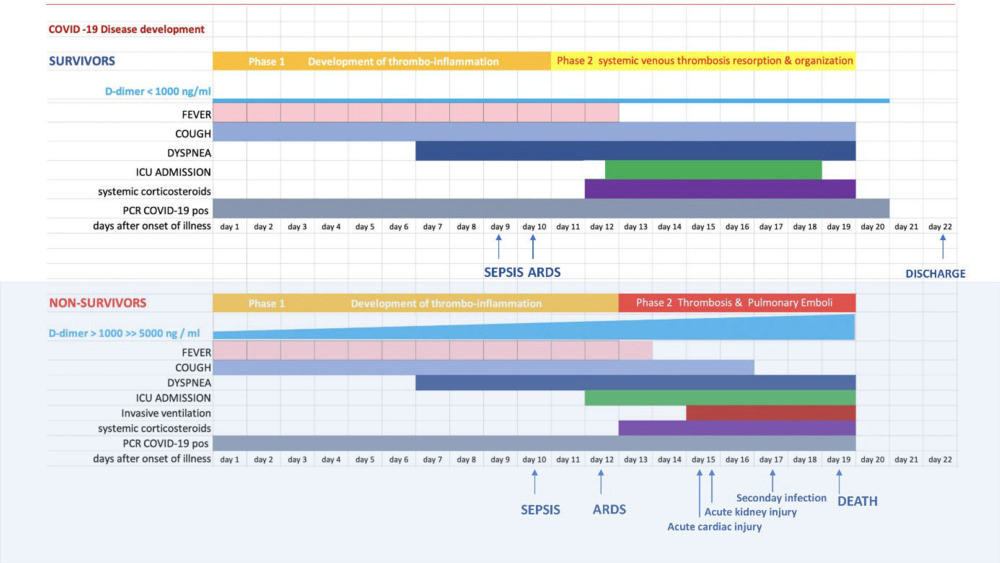
Figure 8. A schematic representation of the pathophysiological disease development of COVID-19, based on the results of the Wuhan population in the context of plasma D-dimer values, clinical and imaging characteristics (Clinical findings reconstructed from reference Zhou et al). (van Beek, et al)
High-res (TIF) version
(Right-click and Save As)
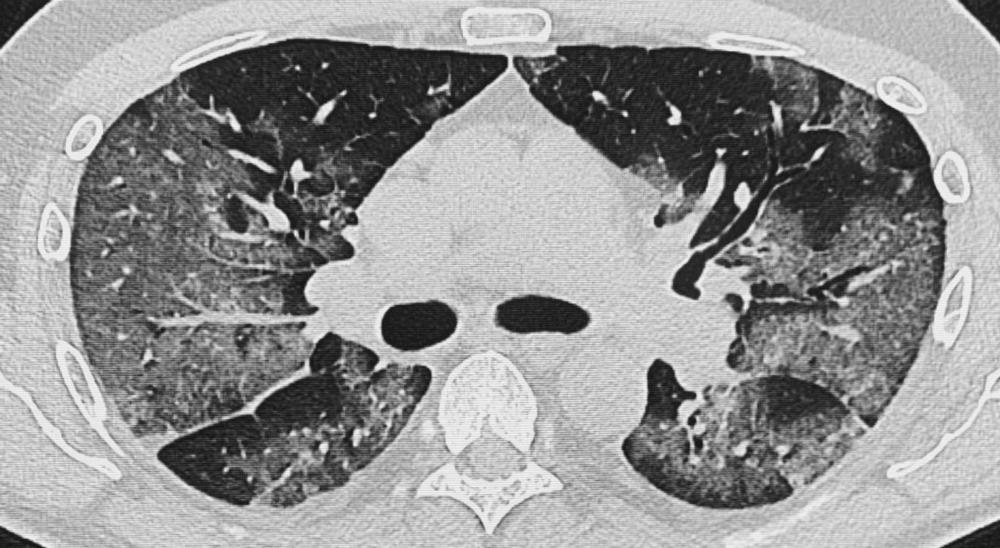
Figure 9. Chest CT images in 51-year-old male patient presenting with progressive symptoms of cough and fever, proven COVID-19. (A) Day 7 after onset of symptoms: CT demonstrates bilateral ground glass opacities (GGOs) and early vascular enlargement. (B) Day 10: Rapid progression of GGOs with vascular thickening and interstitial pulmonary edema. (Figure courtesy of Department of Radiology, Haaglanden Medical Centre, The Hague, the Netherlands.) (van Beek, et al.)
High-res (TIF) version
(Right-click and Save As)
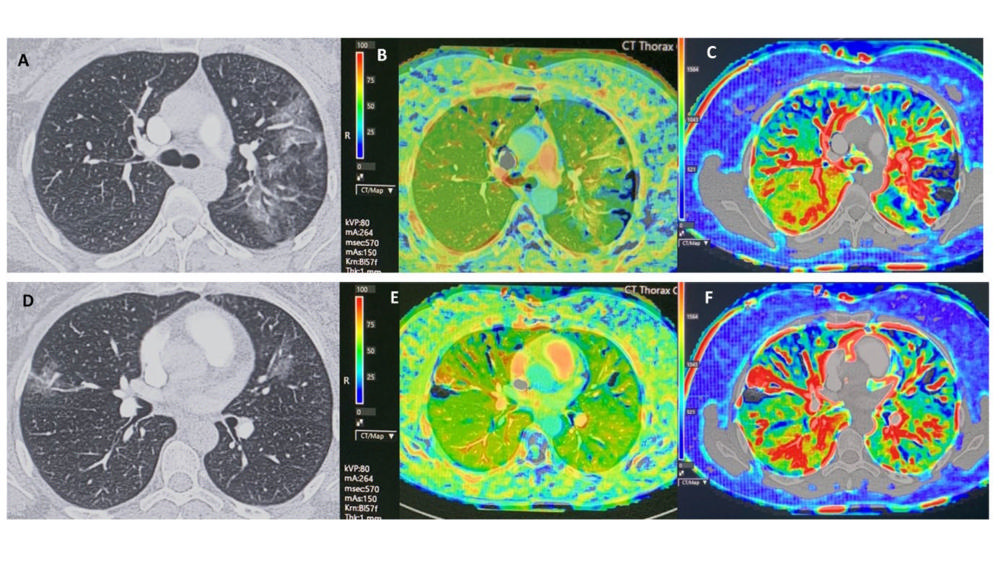
Figure 10. This proof of concept demonstrates CT perfusion findings in phase 1 in a 43-year old female patient proven COVID-19 with multiple ground glass opacities, with plasma D-dimer < 500 ng/ml and without pulmonary embolism on CT pulmonary angiography. There are multiple bilateral perfusion deficits due to microvascular obstruction, with increased blood flow in or adjacent to areas of ground glass opacities. The blood flow is slightly increased in the right lower lobe. Scan parameters: Conventional Dynamic Perfusion CT, Somatom Drive, Siemens; Scan volume 8,4 cm (aorta arch – left atrium); 1 mm recon; Dual input lung perfusion 4D, Vitrea, Vital, Canon; pulmonary flow: ml/min./100 ml; arterial flow: ml/min./100 ml; perfusion Index: % Baseline chest CT findings in selected slices (A, D), with perfusion index (B, E) and pulmonary arterial flow (C, F) in corresponding slices. (Figure courtesy of Department of Radiology, Haaglanden Medical Centre, The Hague, the Netherlands.) (van Beek, et al.)
High-res (TIF) version
(Right-click and Save As)
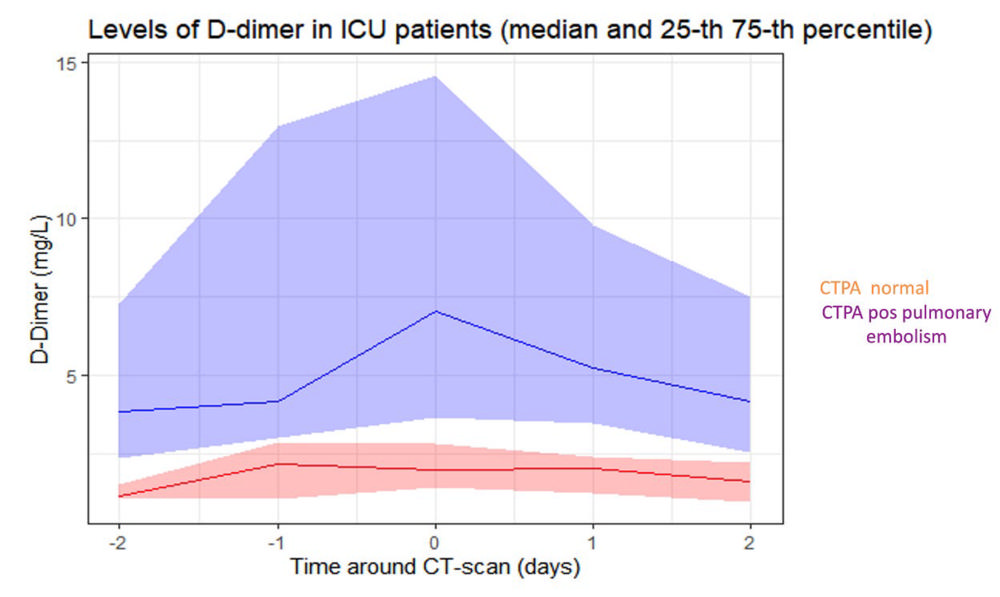
Figure 11. Plasma D-dimer course in 43 consecutive patients with COVID-19, admitted to the intensive care unit. PE was diagnosed with CTPA in 35 patients. (Figure courtesy of Dr Diederik Gommers, Erasmus University Medical Centre, Rotterdam, the Netherlands). (van Beek, et al.)
High-res (TIF) version
(Right-click and Save As)