AI Improves Breast Cancer Risk Prediction
Released: December 17, 2019
At A Glance
- Artificial intelligence can outperform existing models at predicting which women are at future risk of breast cancer.
- Researchers developed a risk model that relies on a deep neural network, a type of AI that can extract information from mammographic images.
- This information has the potential to identify women who would benefit from additional screening with MRI.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Dionna Arnold
1-630-590-7791
darnold@rsna.org
OAK BROOK, Ill. — A sophisticated type of artificial intelligence (AI) can outperform existing models at predicting which women are at future risk of breast cancer, according to a study published in the journal Radiology.
Most existing breast cancer screening programs are based on mammography at similar time intervals—typically, annually or every two years—for all women. This "one size fits all" approach is not optimized for cancer detection on an individual level and may hamper the effectiveness of screening programs.
"Risk prediction is an important building block of an individually adapted screening policy," said study lead author Karin Dembrower, M.D., breast radiologist and Ph.D. candidate from the Karolinska Institute in Stockholm, Sweden. "Effective risk prediction can improve attendance and confidence in screening programs."
High breast density, or a greater amount of glandular and connective tissue compared to fat, is considered a risk factor for cancer. While density may be incorporated into risk assessment, current prediction models may fail to fully take advantage of all the rich information found in mammograms. This information has the potential to identify women who would benefit from additional screening with MRI.
Dr. Dembrower and colleagues developed a risk model that relies on a deep neural network, a type of AI that can extract vast amounts of information from mammographic images. It has inherent advantages over other methods like visual assessment of mammographic density by the radiologist that may not be able to capture all risk-relevant information in the image.
The new model was developed and trained on mammograms from cases diagnosed between 2008 and 2012 and then studied on more than 2,000 women ages 40 to 74 who had undergone mammography in the Karolinska University Hospital system. Of the 2,283 women in the study, 278 were later diagnosed with breast cancer.
The deep neural network showed a higher risk association for breast cancer compared to the best mammographic density model. The false negative rate—the rate at which women who were not categorized as high-risk were later diagnosed with breast cancer—was lower for the deep neural network than for the best mammographic density model.
"The deep neural network overall was better than density-based models," Dr. Dembrower said. "And it did not have the same bias as the density-based model. Its predictive accuracy was not negatively affected by more aggressive cancer subtypes."
The study findings support a future role for AI in breast cancer risk assessment.
"We are not reporting mammographic density currently," Dr. Dembrower said. "In the introduction of individually adapted screening, we use deep learning networks trained to predict cancer rather than taking the indirect route that density offers."
As an additional benefit, the AI approach can continually be improved with exposure to more high-quality data sets.
"Our deep learning experts at the Royal Institute of Technology in Stockholm are working on an update to the model," Dr. Dembrower said. "After that, we aim to test the model clinically next year by offering MRI to the women who stand to benefit the most."
"Comparison of a Deep Learning Risk Score and Standard Mammographic Density Score for Breast Cancer Risk Prediction." Collaborating with Dr. Dembrower were Yue Liu, M.Sc., Hossein Azizpour, Ph.D., Martin Eklund, Ph.D., Kevin Smith, Ph.D., Peter Lindholm, M.D., Ph.D., and Fredrik Strand M.D., Ph.D.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wis., and owned and published by the Radiological Society of North America, Inc. (http://radiology.rsna.org/)
RSNA is an association of over 53,400 radiologists, radiation oncologists, medical physicists and related scientists, promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Ill. (RSNA.org)
For patient-friendly information on mammography and breast cancer screening, visit RadiologyInfo.org.
Images (.JPG and .TIF format)
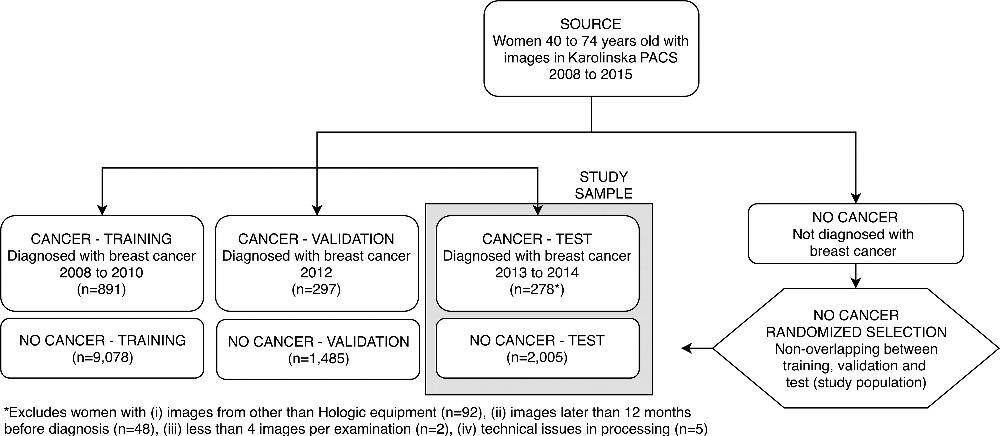
Figure 1. Patient inclusion flowchart shows selection of women in the training and validation samples used for deep neural network development, as well as in the test sample (current study sample). Exclusions are detailed in the footnote. PACS = picture archiving and communication system.
High-res (TIF) version
(Right-click and Save As)
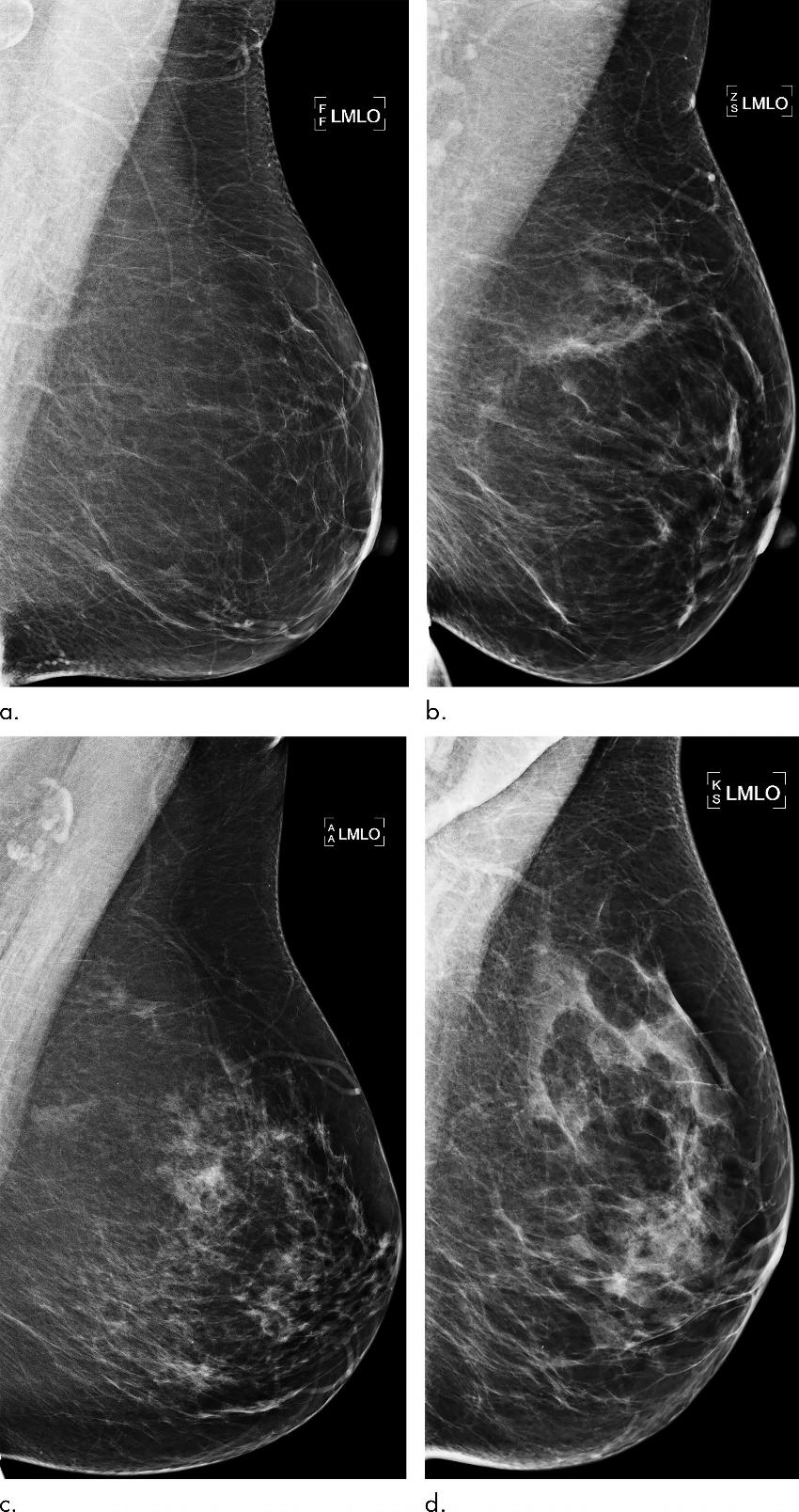
Figure 2. Examples of mammograms with concordance between deep learning (DL) risk score and outcome of breast cancer (true predictions). All images are mediolateral oblique views of left breast. (a) Mammogram in 55-year-old woman with low DL risk score (0.05) who was not diagnosed with breast cancer (ie, true-negative prediction). (b) Mammogram in 47-year-old woman with low DL risk score (0.06) who was not diagnosed with breast cancer (ie, true-negative prediction). (c) Mammogram in 56-year-old woman with high DL risk score (0.30) who received a diagnosis of breast cancer 5.1 years after the examination (ie, true-positive prediction). (d) Mammogram in 57-year-old woman with high DL risk score (0.30) who received a diagnosis of breast cancer 5.0 years after the examination (ie, true-positive prediction). LMLO = left mediolateral oblique.
High-res (TIF) version
(Right-click and Save As)
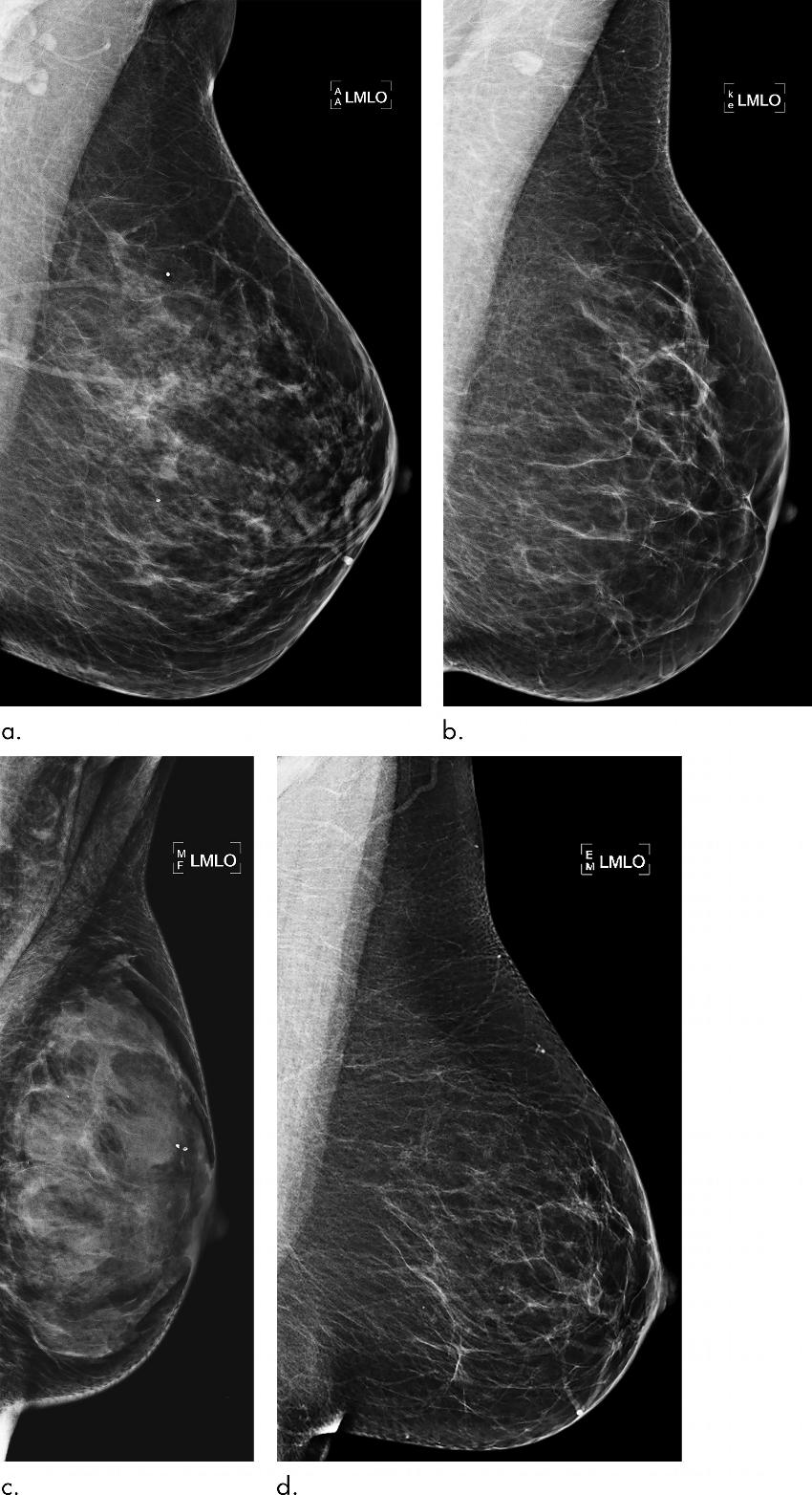
Figure 3. : Examples of mammograms with discordance between deep learning (DL) risk score and outcome of breast cancer (false predictions). All images are mediolateral oblique views of left breast. (a) Mammogram in 44-year-old woman with low DL risk score (0.05) who received a diagnosis of breast cancer 4.7 years after the examination (ie, false-negative prediction). (b) Mammogram in 40-year-old woman with low DL risk score (0.07) who received a diagnosis of breast cancer 4.1 years after the examination (ie, false-negative prediction). (c) Mammogram in 65-year-old woman with high DL risk score (0.53) who was not diagnosed with breast cancer (ie, false-positive prediction). (d) Mammogram in 65-year-old woman with high DL risk score (0.48) who was not diagnosed with breast cancer (ie, false-positive prediction). LMLO = left mediolateral oblique.
High-res (TIF) version
(Right-click and Save As)
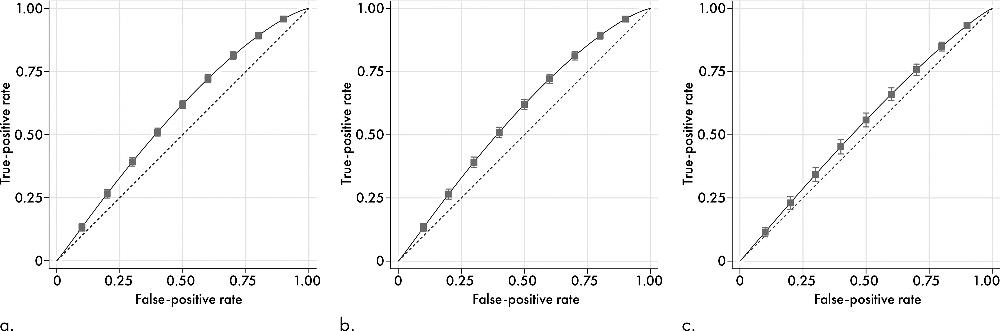
Figure 4. : Receiver operating characteristic curves for the prediction of future breast cancer based on mammographic image evaluation according to three alternative predictors: (a) deep-learning risk score, (b) dense area, and (c) percentage density. Point-wise CIs are shown at every 0.20-increment in false-positive rate.
High-res (TIF) version
(Right-click and Save As)
