Novel Artificial Intelligence Method Predicts Future Risk of Breast Cancer
Released: May 07, 2019
At A Glance
- A new tool using advanced AI methods can predict a woman’s future risk of breast cancer.
- Researchers used almost 90,000 screening mammograms from about 40,000 women to train, validate and test the new breast cancer risk-assessment model.
- The model performs equally well across diverse races, ages and family histories.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Dionna Arnold
1-630-590-7791
darnold@rsna.org
OAK BROOK, Ill. — Researchers from two major institutions have developed a new tool with advanced artificial intelligence (AI) methods to predict a woman’s future risk of breast cancer, according to a new study published in the journal Radiology.
Identifying women at risk for breast cancer is a critical component of effective early disease detection. However, available models that use factors such as family history and genetics fall far short in predicting an individual woman’s likelihood of being diagnosed with the disease.
Breast density—the amount of dense tissue compared to the amount of fatty tissue in the breast on a mammogram—is an independent risk factor for breast cancer that has been added to some models to improve risk assessment. It is based on subjective assessment that can vary across radiologists, so deep learning, a subset of AI in which computers learn by example, has been studied as a way to standardize and automate these measurements.
“There’s much more information in a mammogram than just the four categories of breast density,” said study lead author Adam Yala, Ph.D. candidate at the Massachusetts Institute of Technology (MIT) in Cambridge, Mass. “By using the deep learning model, we learn subtle cues that are indicative of future cancer.”
Yala, in collaboration with Regina Barzilay, Ph.D., an AI expert and professor at MIT, and Constance Lehman, M.D., Ph.D., chief of breast imaging at Massachusetts General Hospital (MGH) in Boston and professor of radiology at Harvard Medical School, recently compared three different risk assessment approaches. The first model relied on traditional risk factors, the second on deep learning that used the mammogram alone, and the third on a hybrid approach that incorporated both the mammogram and traditional risk factors into the deep learning model.
The researchers used almost 90,000 full-resolution screening mammograms from about 40,000 women to train, validate and test the deep learning model. They were able to obtain cancer outcomes through linkage to a regional tumor registry.
The deep learning models yielded substantially improved risk discrimination over the Tyrer-Cuzick model, a current clinical standard that uses breast density in factoring risk. When comparing the hybrid deep learning model against breast density, the researchers found that patients with non-dense breasts and model-assessed high risk had 3.9 times the cancer incidence of patients with dense breasts and model-assessed low risk. The advantages held across different subgroups of women.
“Unlike traditional models, our deep learning model performs equally well across diverse races, ages and family histories,” Dr. Barzilay said. “Until now, African-American women were at a distinct disadvantage in having accurate risk assessment of future breast cancer. Our AI model has changed that.”
“There’s a very large amount of information in a full-resolution mammogram that breast cancer risk models have not been able to use until recently,” Yala added. “Using deep learning, we can learn to leverage that information directly from the data and create models that are significantly more accurate across diverse populations.”
AI-assisted breast density measurements are already in use for screening mammograms performed at MGH. The researchers are tracking its performance in the clinic while working on refining the ways to communicate risk information to women and their primary care doctors.
“A missing element to support more effective, more personalized screening programs has been risk assessment tools that are easy to implement and that work across the full diversity of women whom we serve,” Dr. Lehman said. “We are thrilled with our results and eager to work closely with our health care systems, our providers and, most importantly, our patients to incorporate this discovery into improved outcomes for all women.”
“A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction.” Collaborating with Yala and Drs. Barzilay and Lehman were Tal Schuster, M.S., and Tally Portnoi, B.S.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wis., and owned and published by the Radiological Society of North America, Inc. (http://radiology.rsna.org/)
RSNA is an association of over 54,200 radiologists, radiation oncologists, medical physicists and related scientists, promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Ill. (RSNA.org)
For patient-friendly information on mammography, visit RadiologyInfo.org.
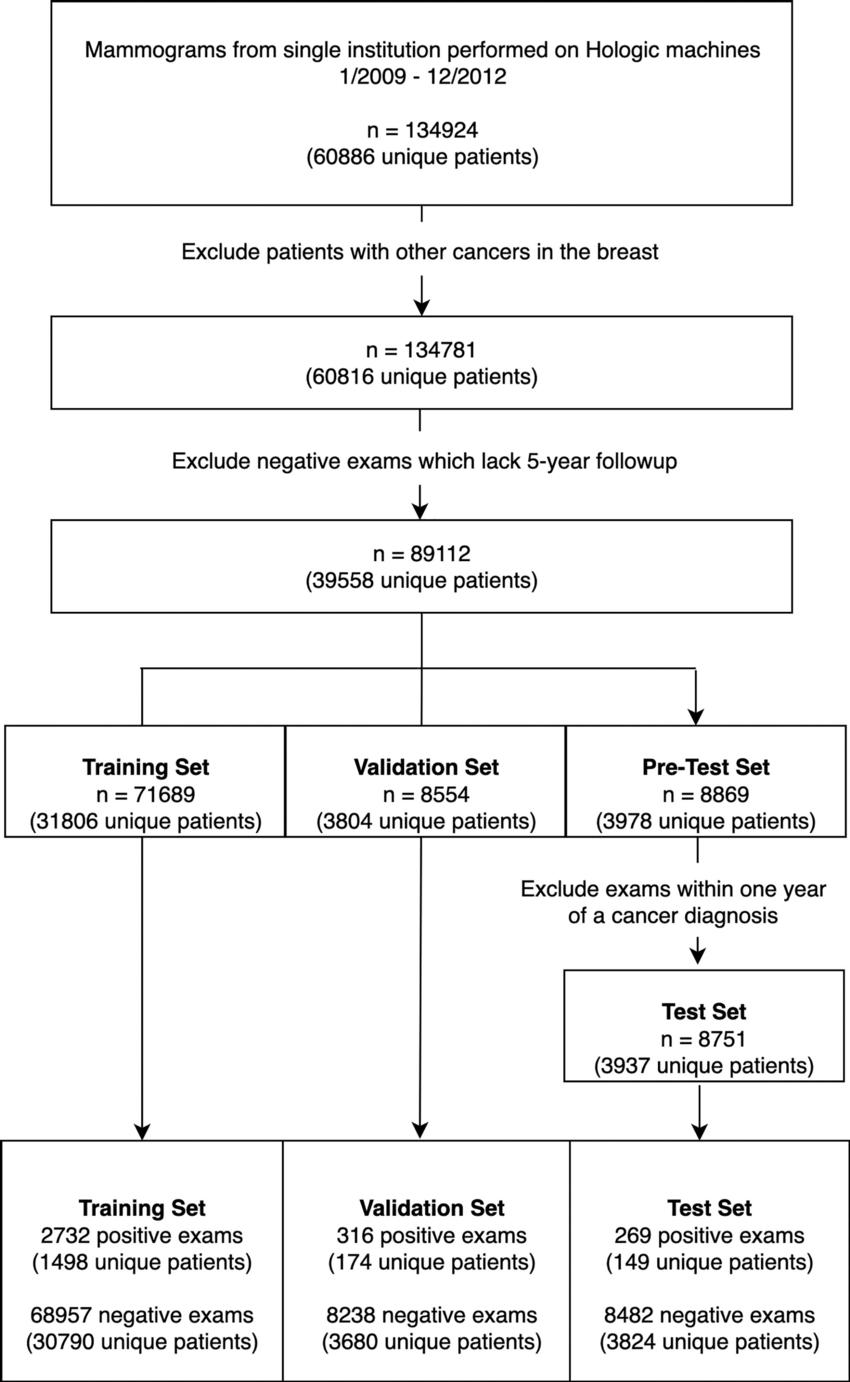
Figure 1. Cohort selection flowchart. There were 134,924 consecutive screening mammograms performed between January 1, 2009, and December 31, 2012. Examinations were defined as positive for cancer if they were followed by a cancer diagnosis within 5 years and negative for cancer if they were not. To restrict the test set to a negative screening population, we excluded examinations that were followed by cancer within 1 year.
High-res (TIF) version
(Right-click and Save As)
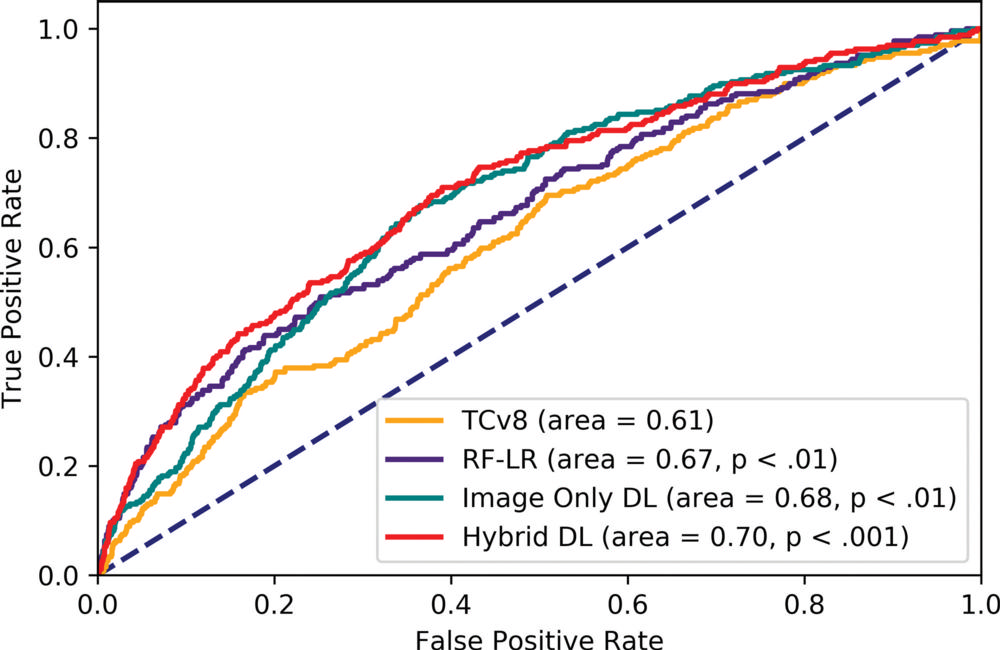
Figure 2. Receiver operating characteristic curve of all models on the test set. All P values are comparisons with Tyrer-Cuzick version 8 (TCv8). DL = deep learning, hybrid DL = DL model that uses both imaging and the traditional risk factors in risk factor logistic regression, RF-LR = risk factor logistic regression.
High-res (TIF) version
(Right-click and Save As)
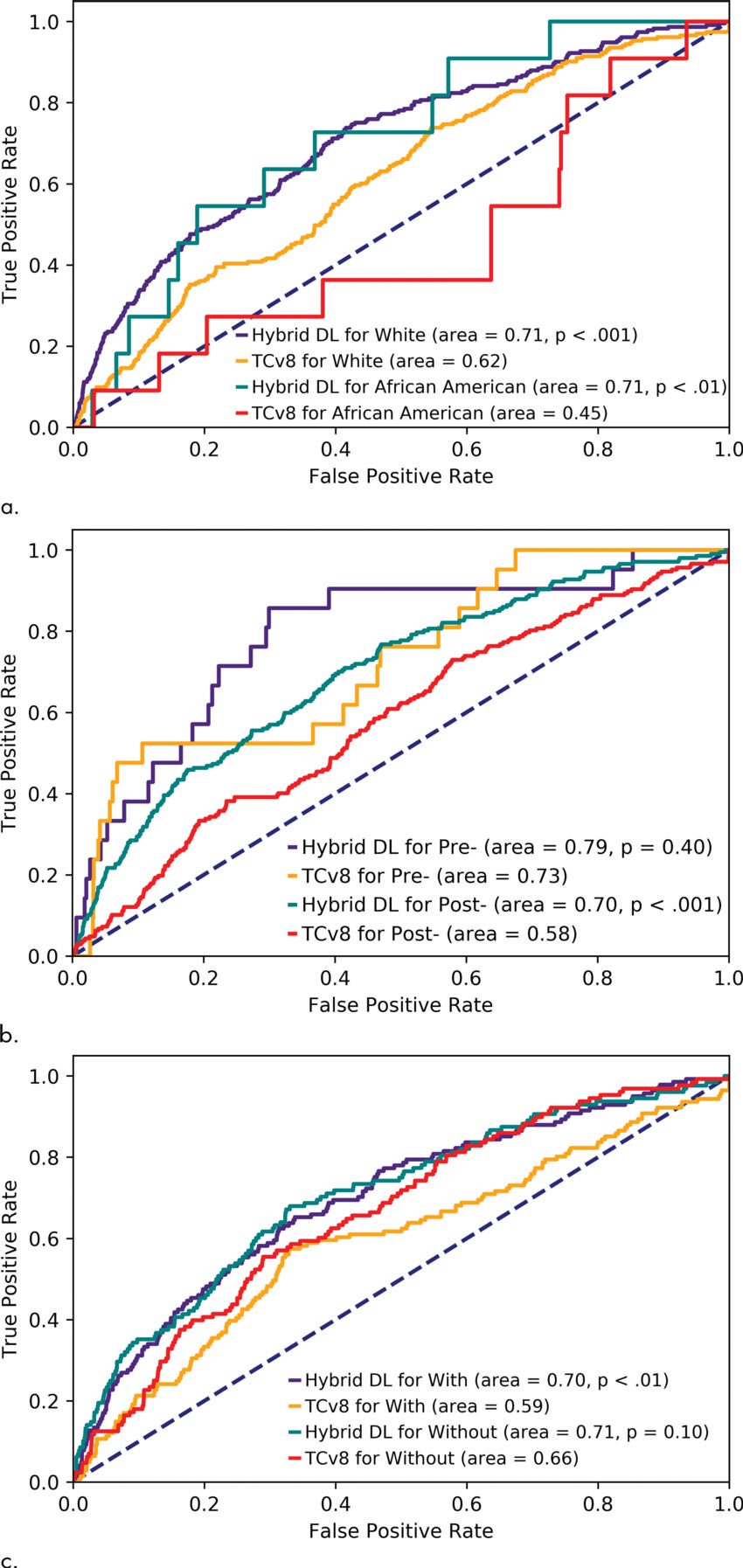
Figure 3. Receiver operating characteristic curve for Tyrer-Cuzick version 8 (TCv8) and hybrid deep learning (DL) for different subgroups of patients: (a) patients who are white and African American, (b) pre- and postmenopausal women, and (c) women with and without any family history of breast or ovarian cancer. All P values are relative to TCv8 for the same subgroup.
High-res (TIF) version
(Right-click and Save As)
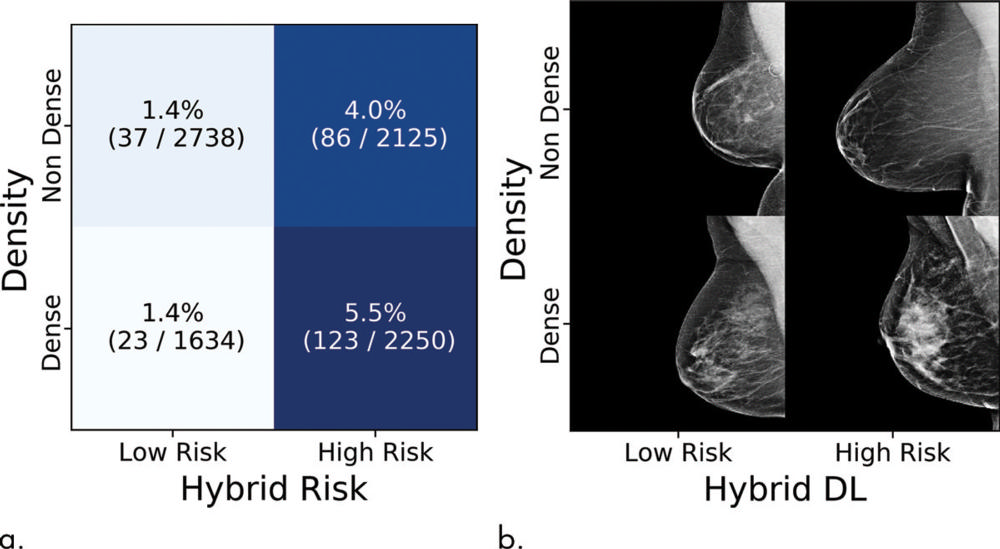
Figure 4. Cancer incidences partitioned by density value and hybrid deep learning (DL) risk assessment. (a) Each tile shows the percent and numerators/denominators of women with examinations within a specific density and risk group who developed cancer within 5 years. (b) Examples of screenings, sampled randomly from all examinations in that group.
High-res (TIF) version
(Right-click and Save As)
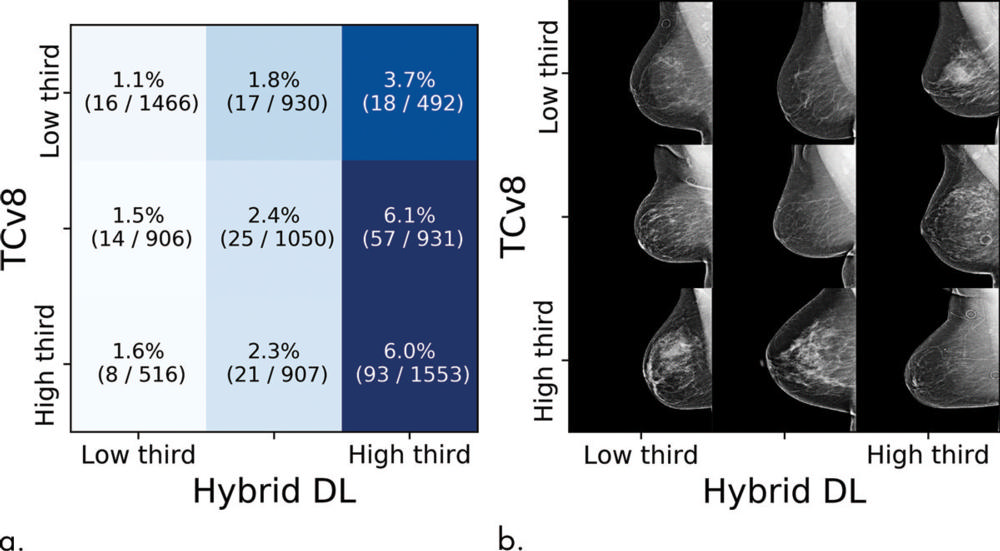
Figure 5. Cancer incidences partitioned by Tyrer-Cuzick risk assessment model (TCv8) and hybrid deep learning (DL) risk assessment. (a) Each tile shows the percent and numerators/denominators of women with examinations within a specific risk range that developed cancer within 5 years. (b) Examples of screenings, sampled randomly from all examinations in that group.
High-res (TIF) version
(Right-click and Save As)


