Iron Measurements with MRI Reveal Stroke’s Impact on Brain
Released: March 12, 2019
At A Glance
- Researchers used an MRI method called R2* mapping to evaluate 181 stroke patients.
- One year after a baseline evaluation, some patients showed higher iron content in a particular brain region, which was associated with worse long-term outcomes.
- The results suggest that R2* mapping may be added to follow-up MRI after stroke to monitor neurodegeneration.
- RSNA Media Relations
1-630-590-7762
media@rsna.org - Linda Brooks
1-630-590-7738
lbrooks@rsna.org - Dionna Arnold
1-630-590-7791
darnold@rsna.org
OAK BROOK, Ill. — A simple MRI method that measures iron content can provide a more comprehensive picture of the consequences of stroke-related damage to the brain, according to a study published in the journal Radiology. Researchers said the findings point to a role for MRI brain iron measurements in monitoring recovery from stroke.
Strokes can result in significant impairments caused by infarctions, or the death of tissue due to the brain’s blood supply being blocked by a clot. Long-term outcomes may also be affected by delayed degeneration of brain areas away from the location of the stroke. One such area is the substantia nigra (SN), a structure of the midbrain that is connected with other parts of the brain in several complex networks.
“Overall, the SN is strongly involved in motor control, but also in regulation of emotions, cognition and motivation,” said study co-author Thomas Tourdias, M.D., Ph.D., professor of radiology at the Centre Hospitalier Universitaire in Bordeaux, France. “Usually, stroke doesn’t directly affect the SN but, by interrupting circuits, stroke can induce secondary degeneration of that area.
” Prior to the new study, this secondary degeneration of SN had only been seen on post-mortem brain examinations. Imaging on a living person has not been able to capture the long-term degeneration, so for the new study, Dr. Tourdias and colleagues employed an MRI technique that measures iron content in the brain. Iron is important for normal brain function, but an excess of it has been linked to neurodegeneration. Iron is released from dying neurons and iron-rich immune cells present around injured neurons. The excess iron further accelerates neurodegeneration through a process called oxidative stress.
The researchers used an MRI technique called R2* mapping to evaluate 181 patients who had suffered a stroke. One year after a baseline evaluation, some of the patients showed higher iron content in the SN. This high iron content was associated with worse long-term outcomes, particularly when it was found on the same side of the brain that the stroke occurred.
“We showed that imaging of iron can also be used to image degeneration remotely from stroke in disconnected areas,” Dr. Tourdias said. “This finding could be clinically useful because it shows that a simple magnetic resonance imaging method such as R2* can provide a more comprehensive picture of the consequences of an infarct.”
The results suggest that R2* mapping may be added to the follow-up MRI after stroke to monitor neurodegeneration, Dr. Tourdias said, by assessing iron levels in the SN. More generally, the findings lend support to the use of iron imaging as a marker for neurodegeneration, an idea that is already being explored for neurodegenerative diseases like Parkinson’s disease. Therapies that prevent iron deposition could potentially be used to slow or stop the degeneration of the neurons.
“We are currently investigating this direction,” Dr. Tourdias said. “Our imaging approach could become crucial in the clinic if modulation of iron develops as a neuroprotective strategy in the future.”
“Neurodegeneration of the Substantia Nigra after Ipsilateral Infarct: MRI R2* Mapping and Relationship to Clinical Outcome.” Collaborating with Dr. Tourdias were Pierre Antoine Linck, M.D., Gregory Kuchcinski, M.D., Fanny Munsch, Ph.D., Romain Griffier, M.D., Renaud Lopes, Ph.D., Gosuke Okubo, M.D., Ph.D., Sharmila Sagnier, M.D., Pauline Renou, M.D., Julien Asselineau, Ph.D., Paul Perez, M.D., Ph.D., Vincent Dousset, M.D., Ph.D., and Igor Sibon, M.D., Ph.D.
Radiology is edited by David A. Bluemke, M.D., Ph.D., University of Wisconsin School of Medicine and Public Health, Madison, Wis., and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology)
RSNA is an association of over 53,400 radiologists, radiation oncologists, medical physicists and related scientists, promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Ill. (RSNA.org)
For patient-friendly information on brain MRI RadiologyInfo.org.
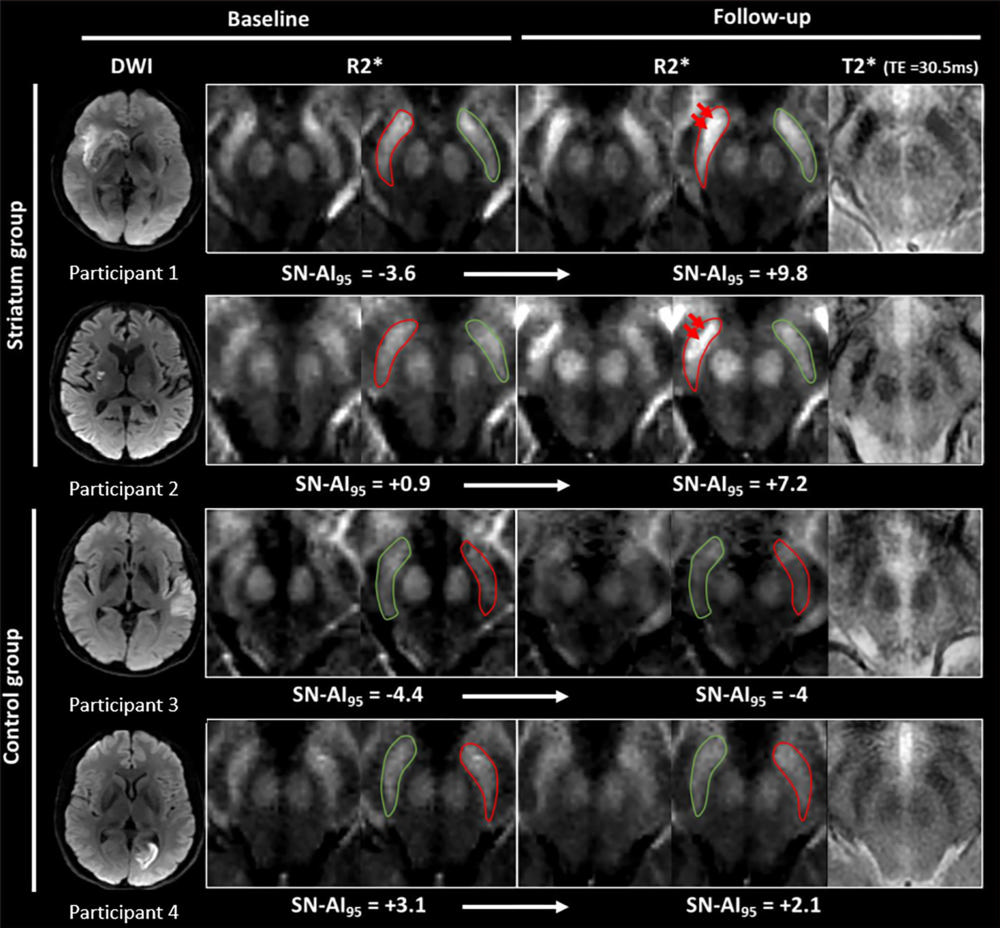
Figure 2. Images show illustrative examples of visual R2∗ modifications within substantia nigra (SN) at baseline (24–72 h) and follow-up (1 y) in striatum (participants 1 and 2) and control groups (participants 3 and 4).
High-res (TIF) version
(Right-click and Save As)
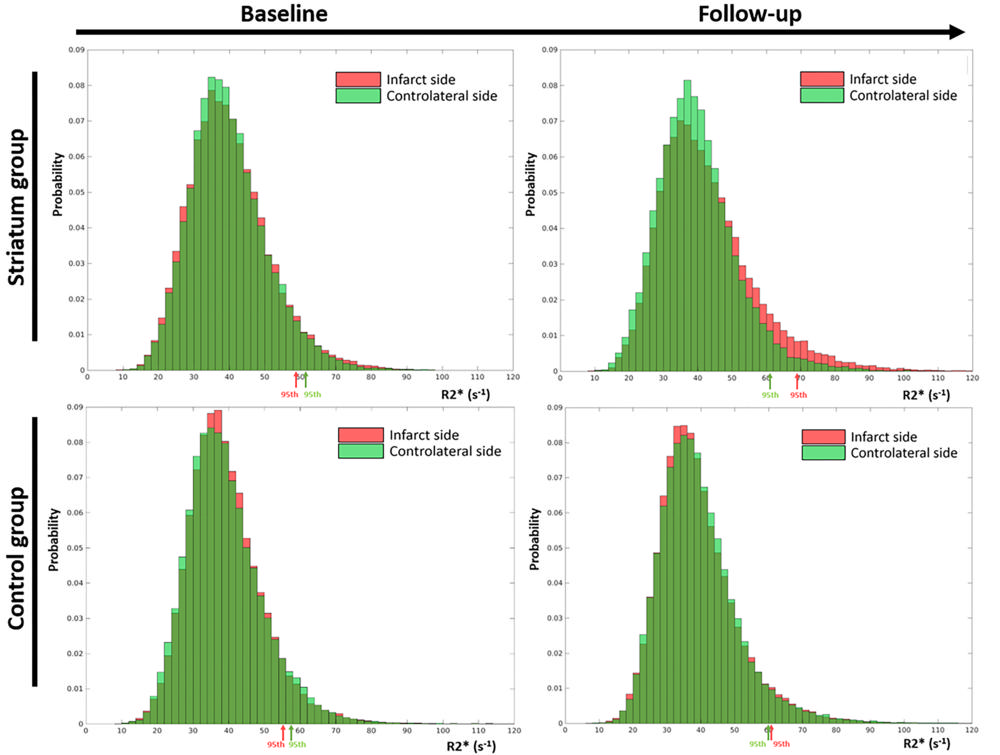
Figure 3. Cumulative histograms show R2∗ values measured within substantia nigra (SN) of participants from striatum (n = 75) and control (n = 106) groups at baseline (24–72 h) and follow-up (1 y). Histograms from R2∗ values measured within SN ipsilateral to infarcts are in red and histograms from R2∗ values measured within SN contralateral to infarcts are in green. Locations of 95th percentile of R2∗ are indicated by red and green arrows. Distribution of R2∗ values within SN ipsilateral to infarcts involving striatum is skewed to right with higher 95th percentile at 1-year MRI.
High-res (TIF) version
(Right-click and Save As)

Figure 4. Average R2∗ maps (axial plane) in striatum (n = 75) and control (n = 106) groups at baseline (24–72 h) and follow-up (1 y). All infarcts were flipped on left side. Color-coded R2∗ values for striatum group and control group are shown at baseline and follow-up. Lower rows show voxel-based comparison of R2∗ values between striatum and control groups at baseline and follow-up.
High-res (TIF) version
(Right-click and Save As)
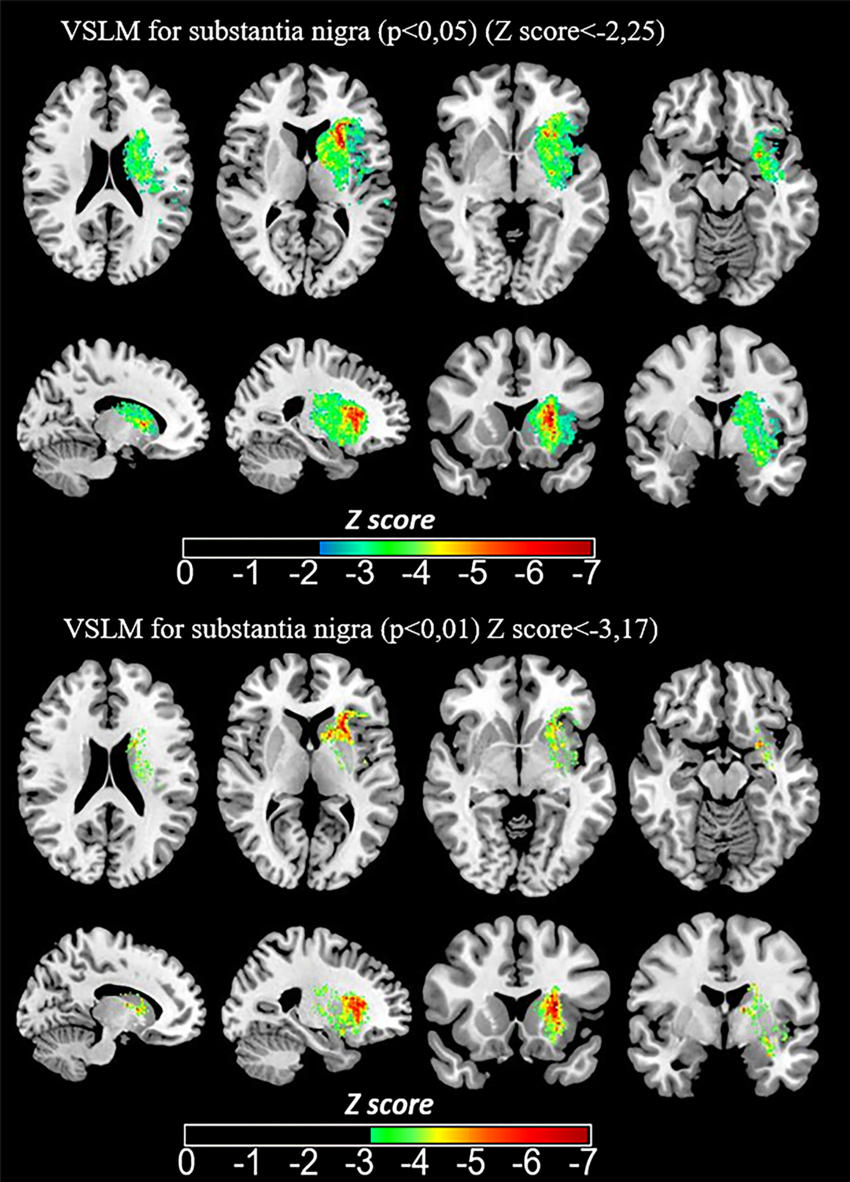
Figure 5. Images show voxel-based lesion-symptom mapping (VLSM) quantified impact of baseline infarct location on SN-AI95 of R2∗ at 1-year follow-up. Z score resulting from Brunner-Menzel testing is indicated by color range and is overlaid on three-dimensional T1-weighted image normalized to Montreal Neurological Institute 152 space atlas in axial, sagittal, and coronal planes. Association between infarct voxels and higher SN-AI95 is shown by lower Z score.
High-res (TIF) version
(Right-click and Save As)